This article is a review and breakdown of a recent study. The study reviewed is The Concept of Skeletal Muscle Memory: Evidence from Animal and Human Studies by Snijders et al. (2020)
Key Points
- Evidence is fairly clear that muscle fibers accrue more myonuclei as they grow enough.
- Whether myonuclei are lost following muscle fiber atrophy is less clear. Some studies show a loss of myonuclei, while others show that myonuclei are retained.
- The only direct evidence we have for myonuclei-mediated muscle memory comes from rodent studies.
- Retention of myonuclei following muscle atrophy may contribute to muscle memory, but the relationship isn’t as straightforward as it’s often presented.
Muscle fibers are very large individual cells, and unlike smaller cells types that have a single nucleus, muscle fibers have multiple nuclei, referred to as myonuclei. It’s thought that myonuclei can only “oversee” a finite, relatively fixed volume of sarcoplasm (the “stuff” inside muscle fibers); the sarcoplasm overseen by a particular myonucleus is its “myonuclear domain.” As fibers get larger, and myonuclear domains approach their limits, muscle fibers must gain new myonuclei from surrounding cells (satellite cells) to continue growing. This collection of observations is often referred to as “myonuclear domain theory.”
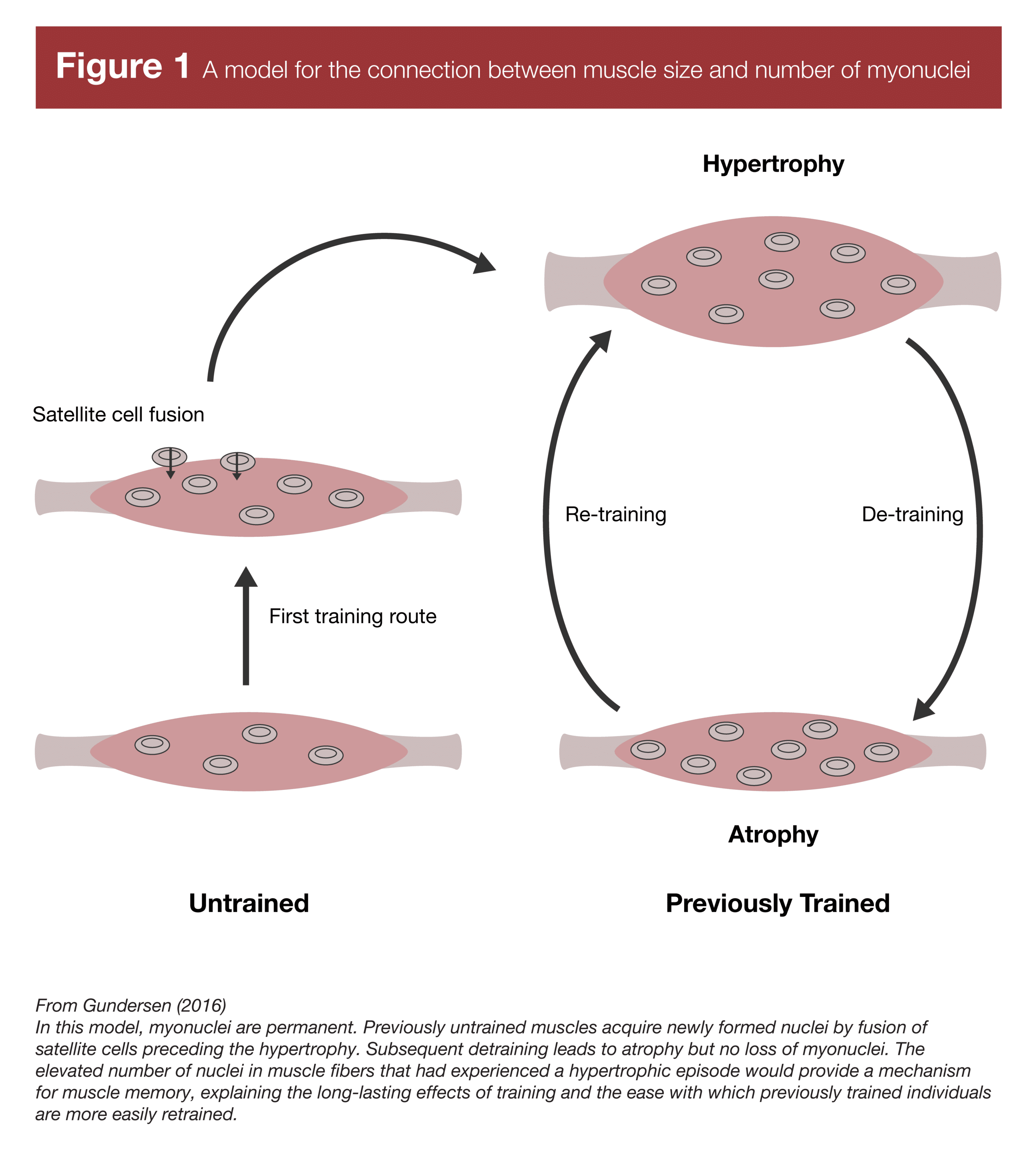
While muscle fibers must gain more myonuclei to continue growing, it’s not clear that they lose myonuclei when they atrophy. A groundbreaking 2013 study found that mice gained muscle and accrued more myonuclei when given testosterone (2). When testosterone treatment was removed, the mice lost muscle tissue, but didn’t lose myonuclei. Following a period of tension overload, the mice that had previously received testosterone gained muscle faster than a control group of mice that did not have elevated levels of myonuclei. This finding (in addition to other studies that had observed no loss in myonuclei in different animal atrophy models) led people to theorize that myonuclei aren’t lost upon detraining and atrophy, leading to elevated myonuclear density; increased myonuclear density, in turn, allowing for faster muscle hypertrophy. Each myonucleus has a finite “transcriptional capacity” (the amount of protein it can direct ribosomes to build), so a greater density of myonuclei following detraining allows muscle tissue to be re-built faster than it can be built originally. This mechanism is thought to underpin the observation of “muscle memory” – rapid regain of muscle following a layoff from training. I’m sure most people have seen a version of this iconic image (Figure 1) illustrating this concept (3).
This is a nice narrative, but how strong and consistent is the evidence for it? As it turns out, many people (myself included) may have gotten too excited, too soon. The presently reviewed publication (1) examines the animal and human evidence for myonuclear permanence (not losing myonuclei following muscle atrophy) and myonuclei-facilitated muscle memory. The evidence for both of these concepts is somewhat weak and inconsistent, though I think the general concept may still have some degree of merit.
Purpose and Hypotheses
Purpose
The purpose of this review was to, “showcase any evidence for the presence or absence of the proposed ‘muscle memory’ in both animal and human models of muscle fiber atrophy and hypertrophy.” Since this was a review article, there were no hypotheses.
Subjects and Methods
Subjects
The “subjects” in the present review were the findings of previous studies. There were 60 findings from animal studies and 16 findings from human studies included in the review.
Experimental Design
This was not a systematic review, which means that there wasn’t a pre-specified search strategy for identifying potential studies to include, nor were there defined inclusion and exclusion criteria. However, in this particular instance, I don’t think that’s a huge drawback. This particular niche of muscle physiology is pretty small, so I suspect the authors of this review simply knew of all of the relevant studies in the area. The fine folks at Maastricht University that authored this review certainly know this area of the research better than I do, but for what it’s worth, I’m not aware of any significant papers in this niche that weren’t included in this review (1). I do think it’s essentially a systematic review, for all intents and purposes (not in form, but in function).
The review itself starts by explaining the research methods people have used to study myonuclei, and the pros and cons of those methods. Then, it explains how we arrived at our current conception of myonuclei-mediated muscle memory. Finally, it summarizes the evidence for and against myonuclei-mediated muscle memory in animal and human studies.
Findings
Research Methods
Since MASS isn’t a review targeted at muscle physiologists, I won’t explain the methods used to assess myonuclei in excruciating detail. However, it’s worth noting that there’s one major issue associated with studying myonuclei: Care needs to be taken to ensure myonuclei are distinguishable from other nuclei (like satellite cell nuclei). The consensus seems to be that there are two ideal methods for studying myonuclei. The first method involves staining muscle fibers with dyes that allow you to distinguish myonuclei, cell borders, and satellite cells. The second method involves using gene transfer to label myonuclei with a fluorescent green protein, and assessing the same region of muscle fibers over time in vivo (i.e. in a living animal, without removing tissue). The second method is arguably better than the first, because there’s no guarantee that muscle cross-sections are going to be perfectly consistent; when you’re simply observing the same fibers and same myonuclei over time, you eliminate that drawback. However, both of these methods are still relatively new, so studies using them comprise a minority of the total research in the area.
History
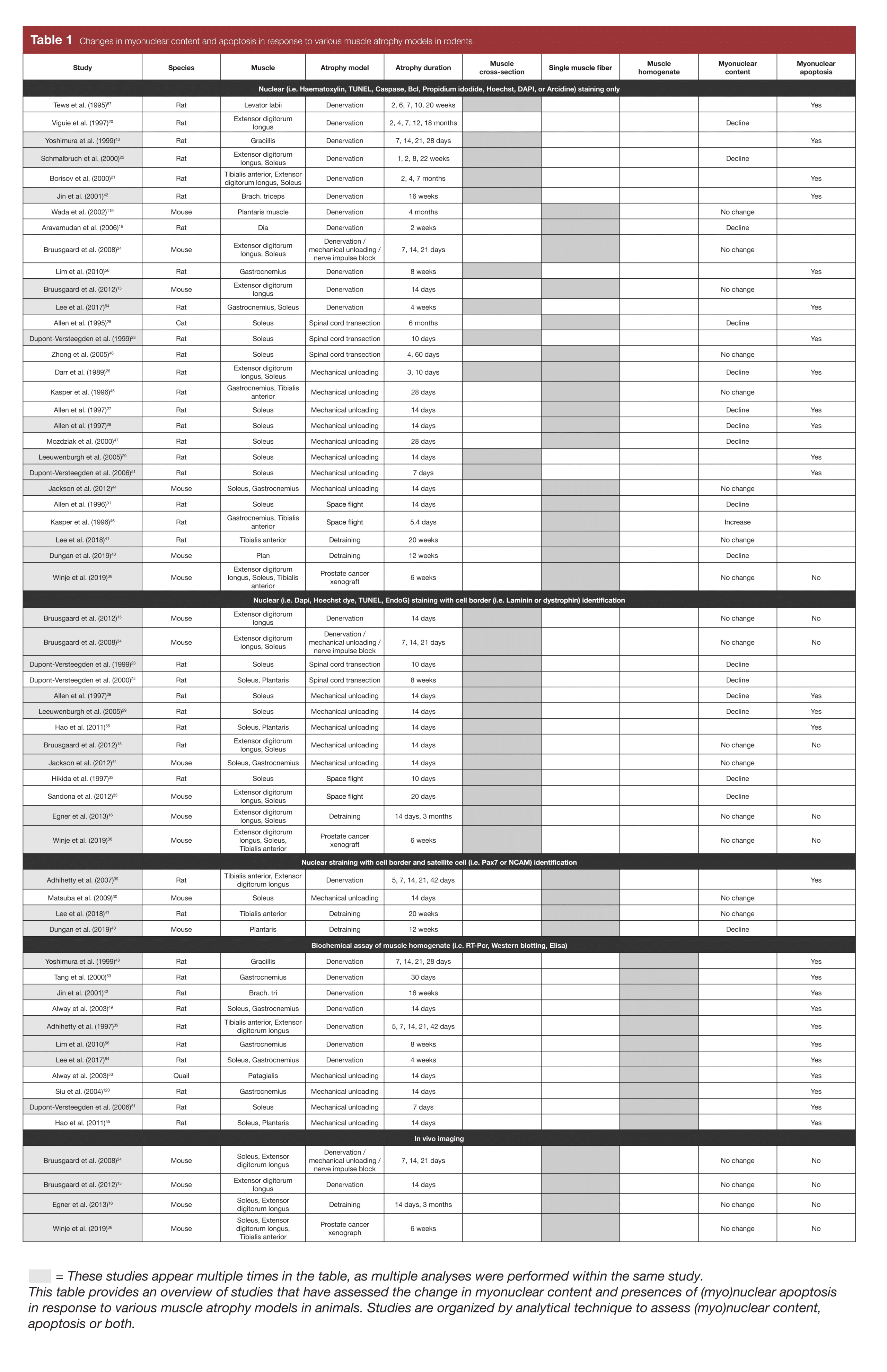
The history of this line of research is interesting. I didn’t become aware of myonuclei research until after the concept of myonuclei-mediated muscle memory was already reasonably popular. However, the work that largely established that idea was published in 2012 and 2013 by Bruusgaard, Egner, and Gundersen (2, 4). Prior to that point, it was generally believed that muscle fibers gained myonuclei when they grew, and lost myonuclei when they atrophied. And in fact, there was good reason to believe that to be the case. Many studies had found evidence of nuclear apoptosis (death) during atrophy, and a good chunk of the early studies in the area did find that the total number of nuclei present in a muscle cross-section decreased as muscle fibers decreased in size (summarized in Table 1). However, the groundbreaking studies in 2012 and 2013 used the in-vivo method of myonuclear assessment mentioned above, which clearly delineates between myonuclei and the nuclei of other cells in the area. The authors of those studies argued that previous studies were merely observing a loss in other nuclei (like satellite cell and stromal cell nuclei), not myonuclei. After those seminal studies were published, the popular conception of myonuclei research started changing – myonuclei are accrued when muscle fibers grow, but they aren’t lost when fibers atrophy. Thus, by this interpretation, myonuclei you accrue are effectively permanent, providing a mechanism for rapid muscle regrowth following atrophy.
Animal Research
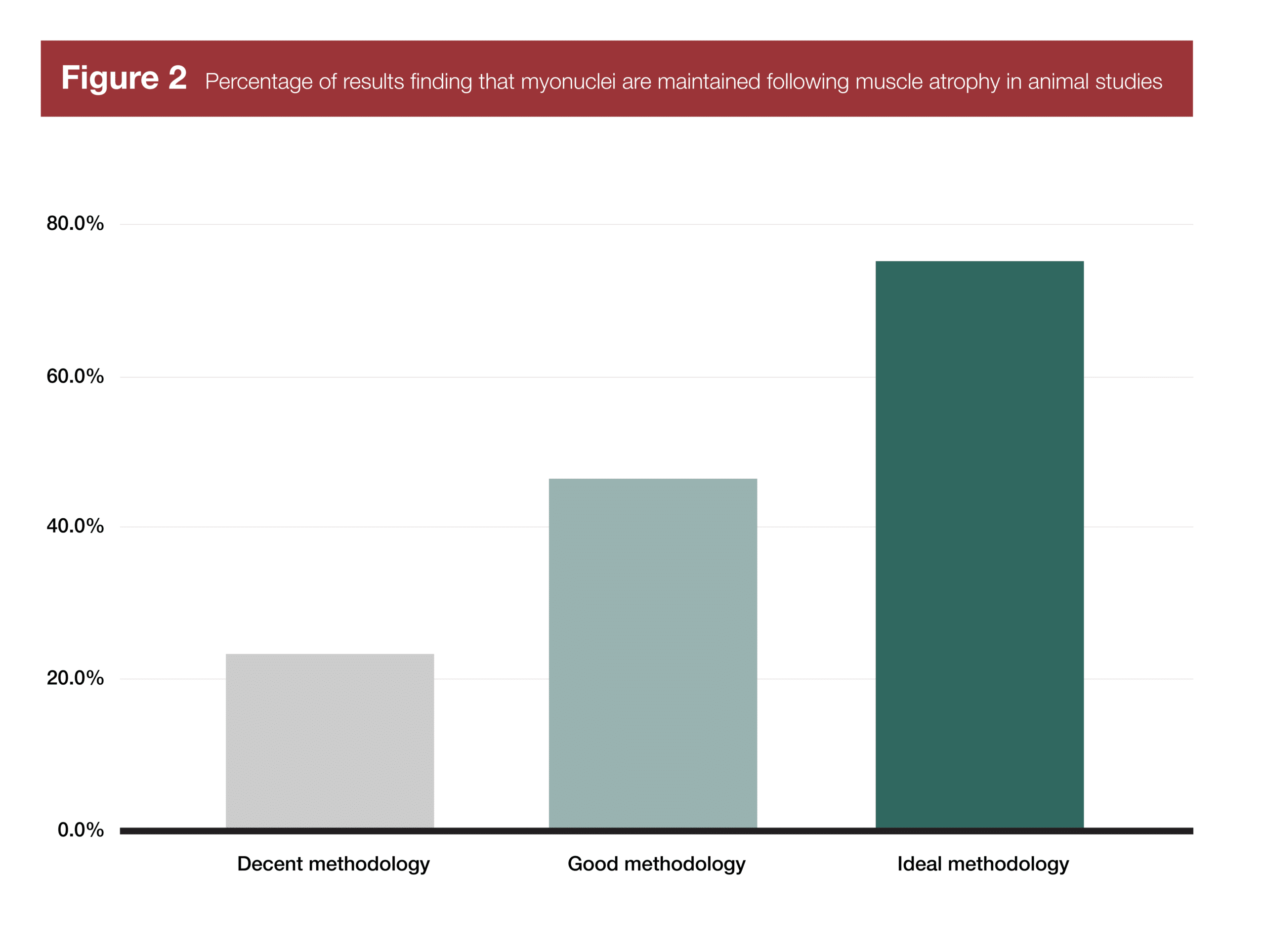
With the exception of one study on quail and one study on cats, all of the animal-based myonuclei research has been conducted on mice and rats. Out of 60 research findings, 39 used methods that are now known to be problematic (only using nuclear staining, or running assays on muscle homogenate), due to difficulties distinguishing between myonuclei and the nuclei of other cells in the area. Of those 39 studies, 30 found evidence that nuclei were lost following muscle atrophy. 34 of those 39 studies were carried out before 2013, which explains why the field initially believed that myonuclei were lost following muscle atrophy; they didn’t know the methods being used were problematic, and since there was plentiful evidence that nuclei were being lost, what were the odds that all myonuclei were being spared?
There are 13 studies that are a step up methodologically, but still don’t use ideal methods. In these studies, fibers are stained for both nuclei and the cell membrane (sarcolemma). All nuclei inside a fiber are myonuclei, and all of the nuclei outside a fiber are not myonuclei. I’ve never looked down a microscope and tried to count myonuclei, but apparently this method still leaves you with some judgement calls, since most nuclei are clustered around the sarcolemma. In these 13 studies, the research is almost perfectly split; seven studies report a loss of myonuclei following muscle atrophy, while six studies find that myonuclei are maintained following atrophy.
Finally, just eight studies either use the ideal histological approach (staining for myonuclei, satellite cells, and cell membranes), or assess myonuclei in vivo. In the four studies using a histological approach, two report a loss of myonuclei following atrophy, and two report no change. In the four studies using in vivo imaging, none report a loss of myonuclei.
Thus, as research methods improve, more and more studies fail to find a decrease in myonuclei following atrophy. As such, it’s likely that during muscle atrophy, satellite cells and stromal cells decrease in density, but myonuclei are more likely to stick around. However, there are studies that still indicate that myonuclei are lost following muscle atrophy, even when using high-quality methodology, which suggests that myonuclei aren’t truly permanent, though they’re probably lost at a slower rate than the rate at which atrophy occurs.
Cross-Sectional Human Research
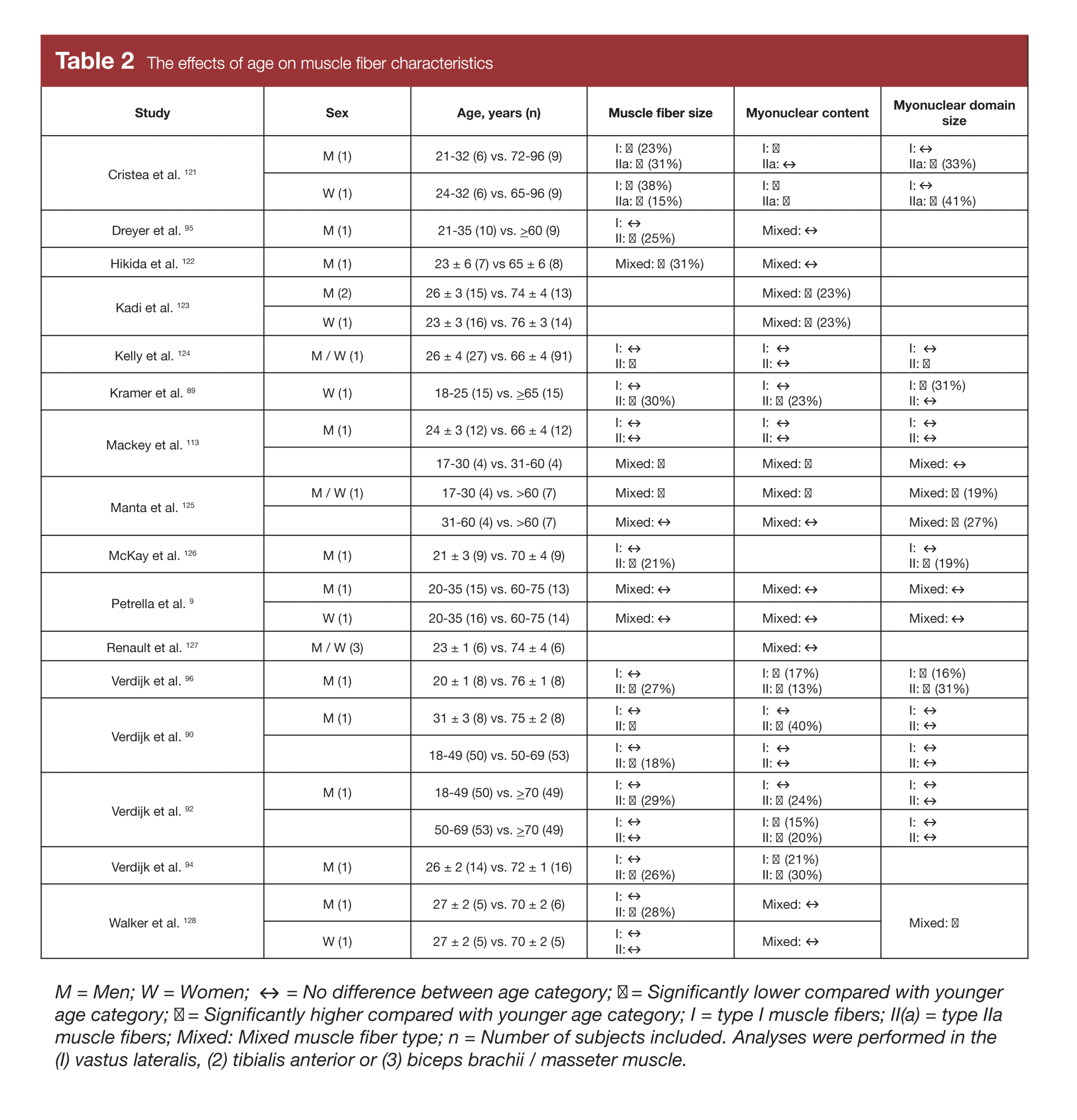
The vast majority of the human research in this area is cross-sectional. Rather than induce muscle atrophy, researchers compare muscle fiber size, myonuclear content, and myonuclear domain size between young and older adults. Older adults (60+ years old) generally have smaller muscle fibers than younger adults – especially type II fibers. If a study finds that older adults have smaller fibers than young adults but similar myonuclear content, and thus smaller myonuclear domains, that suggests that myonuclei weren’t lost following age-related muscle atrophy.
The evidence is mixed. I’ll ignore findings specific to type I fibers (for the most part, younger and older adults have type I fibers of similar size with similar myonuclear content and similar myonuclear domain sizes), but of the groups where type II fibers were assessed, four found that myonuclear domains were smaller in older adults, while seven found that myonuclear domains were similar sizes in young and older adults. In studies that didn’t assess type I and type II fibers separately, five found that myonuclear domains were smaller in older adults, four found that myonuclear domains were similar sizes in young and older adults, and two actually found that myonuclear domains were larger in older adults.
On balance, the picture painted by these studies is fairly similar to the picture painted by animal research. 9 of 22 findings suggest that myonuclei are either preserved or lost at a rate that’s slower than the rate of fiber atrophy, 11 suggest that fiber atrophy and myonuclei loss occur in similar proportions, and 2 suggest that myonuclei are lost to a greater extent than atrophy occurs. The two age-based cohorts in most of these studies are separated by 40-50 years, so the fact that almost half of the studies find that myonuclear domains are larger in the older cohort may suggest that, while myonuclei can be lost during the atrophy process, they may not be lost at quite the same rate as atrophy occurs. However, these studies also suggest that “myonuclear permanence” probably does not occur – over a time span of decades, some degree of myonuclear apoptosis probably does occur. Note that I’m using guarded language, however. Since these studies are cross-sectional in nature, we can’t necessarily assume causation, and we also can’t necessarily assume that muscle atrophy due to aging has the same effect on myonuclei as muscle atrophy due to detraining.
Longitudinal Human Research
There are four longitudinal studies worth mentioning. Only one attempted to replicate the mouse study demonstrating myonuclei-related muscle memory, but all four are relevant to the proposed myonuclei-mediated muscle memory model.
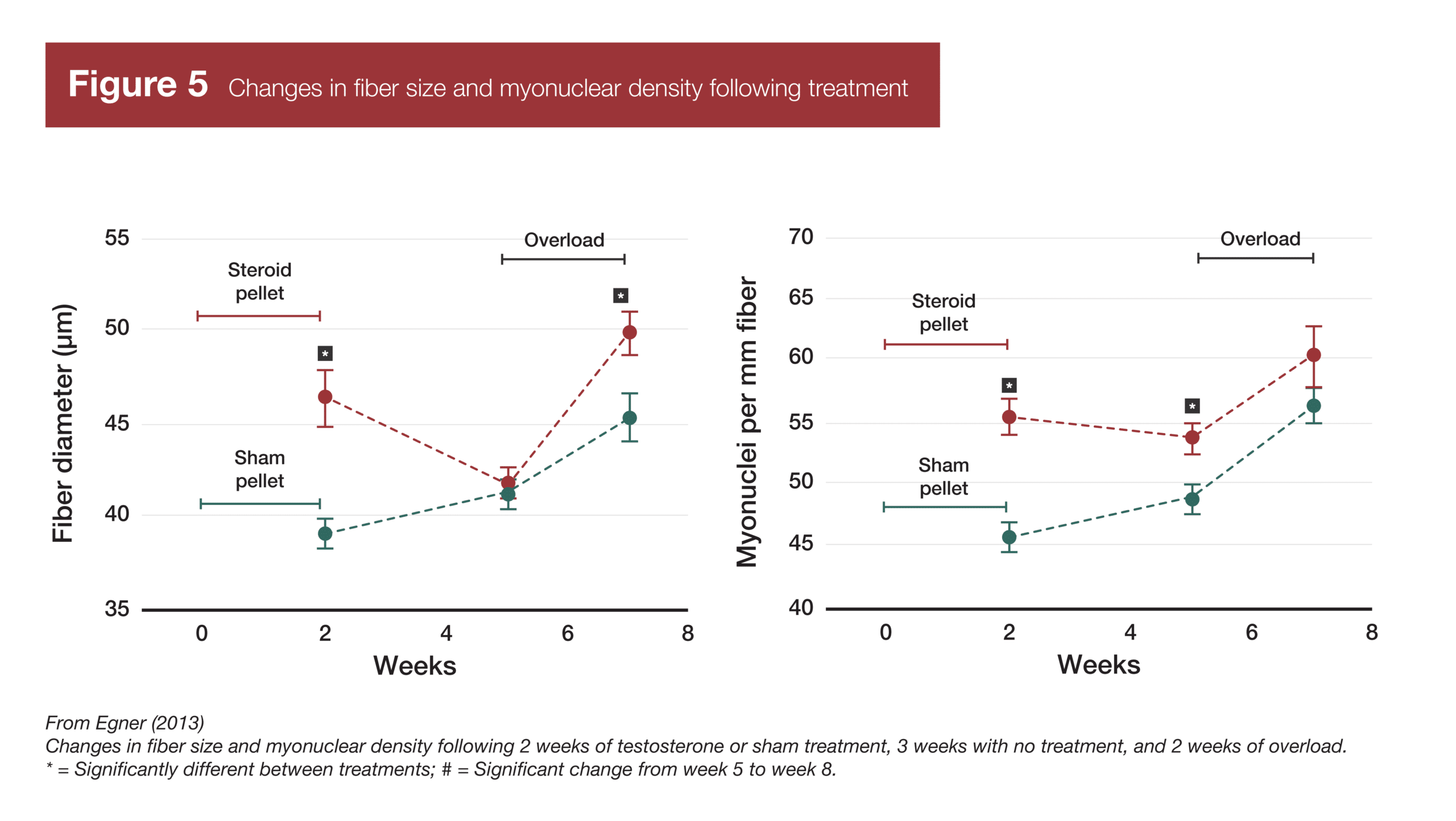
As mentioned in the intro, a 2013 study by Egner et al found evidence of myonuclei-related muscle memory in mice (2). They administered testosterone to the mice, which induced both muscle growth and myonuclear addition. Then, testosterone administration was withdrawn, and the mice lost muscle, returning to their baseline levels of muscularity. However, myonuclei content remained elevated in spite of muscle atrophy. Then, the original group of mice underwent a 14-day overload period (induced by synergist ablation), along with another group of mice that had not received testosterone treatment and had not accrued additional myonuclei. The group of mice that had previously received testosterone treatment and accrued more myonuclei experienced twice as much hypertrophy as the group of mice that had not previously received testosterone treatment.
A recent study by Psilander and colleagues attempted to replicate Egner’s results (5). Instead of using a control group, they used a within-subject design, and hypertrophy was initially induced via resistance training instead of testosterone administration. Subjects trained one leg for 10 weeks, detrained for 20 weeks, and then trained both legs for 5 weeks. However, Psilander’s study suffered from two major issues: While muscle fibers in the trained leg grew during the initial 10 weeks of training, the subjects didn’t accrue more myonuclei, and the fibers didn’t atrophy back to their initial size during the 20 week detraining period. This wasn’t Psilander’s fault, naturally, but it rendered the study unable to actually assess myonuclei-related muscle memory since neither myonuclear accretion nor detraining-induced fiber atrophy went according to plan.
Another study in older adults had more success with manipulating myonuclei content (6). It involved six months of training and one year of detraining. It wasn’t designed to fully test the idea of muscle memory because it didn’t include a retraining period, but it did test the first part of the theory of myonuclei-related muscle memory – gaining myonuclei following resistance training, and not losing those myonuclei following muscle atrophy. Subjects did experience hypertrophy and an increase in myonuclear content during the six-month training period, but following a year of detraining, both muscle fiber size and myonuclear content had regressed back to baseline.
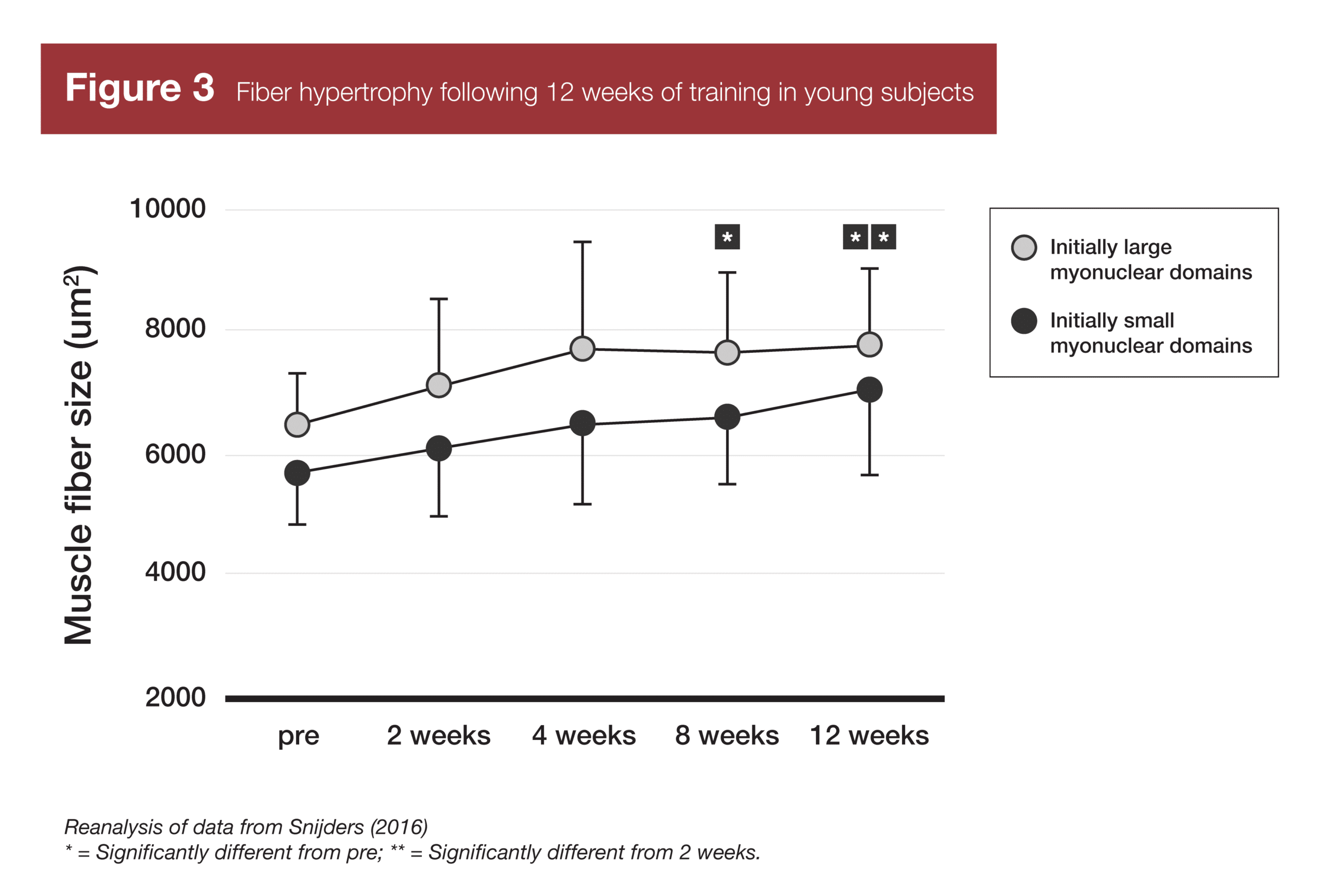
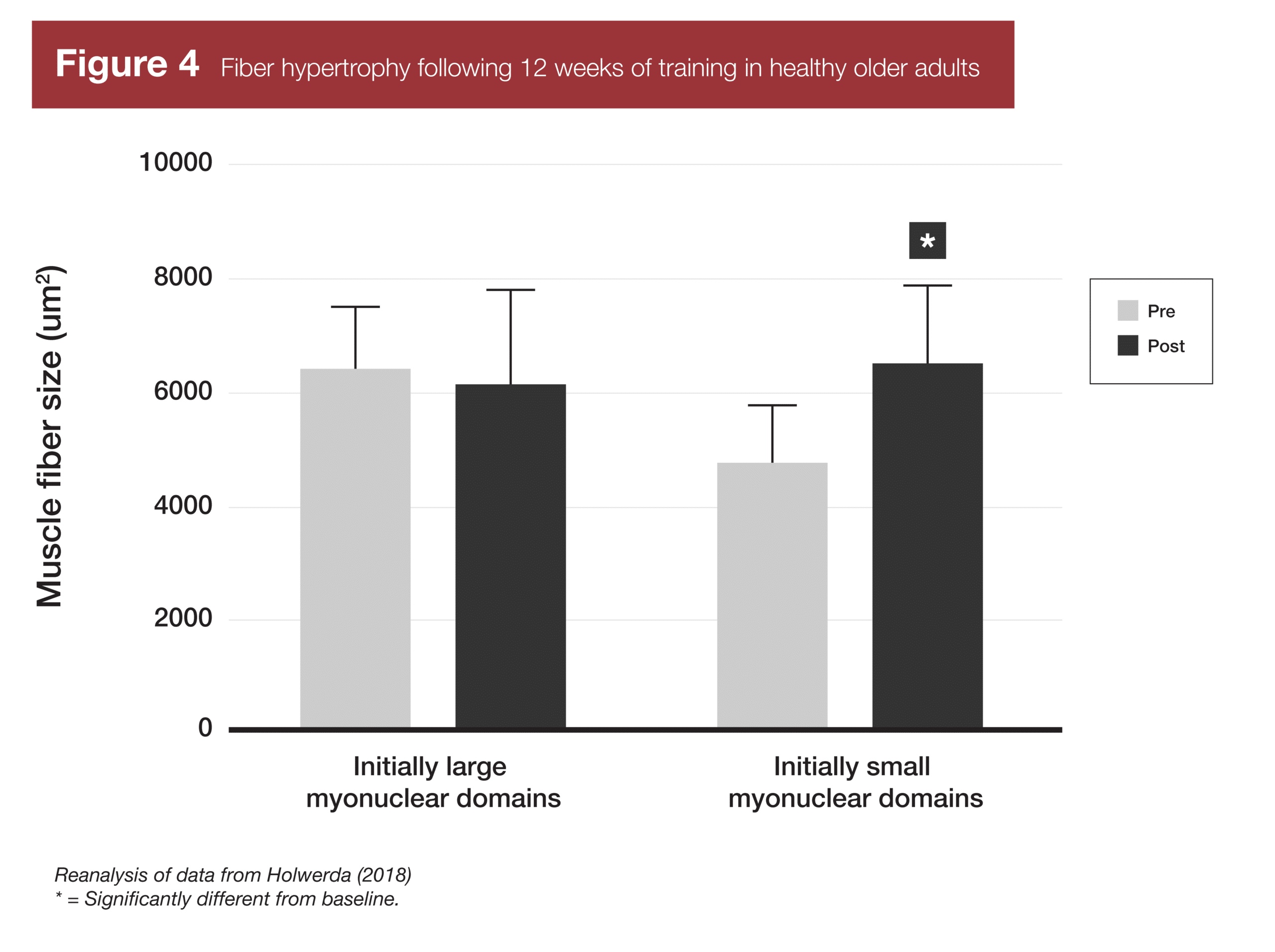
Finally, two studies have provided evidence related to the last part of the myonuclei-related muscle memory concept – if your myonuclear domains are smaller, you’ll build muscle more rapidly due to enhanced transcriptional capacity. A study in younger adults (7) found that subjects with smaller myonuclear domains at baseline did not gain muscle faster than subjects with larger myonuclear domains at baseline. The subjects with larger myonuclear domains at baseline simply accrued new myonuclei faster, and were thus able to build muscle at the same rate. However, a similar study in older adults (8) did find that subjects with smaller myonuclear domains at baseline experienced significant increases in muscle fiber size following training, while subjects with larger myonuclear domains failed to experience significant hypertrophy.
Interpretation
To start this section, I want to make one thing clear: The question here isn’t, “is ‘muscle memory’ real,” but rather, “does this particular mechanism seem to be a primary driver of the ‘muscle memory’ phenomenon?” The phenomenon of “muscle memory” – regaining muscle post-atrophy faster than building it initially – was first scientifically documented in the early 90s (9), and both research (10) and thousands of lifers’ anecdotes testify to its existence. The issue at hand is whether myonuclear permanence (not losing myonuclei following muscle atrophy) a) is a real phenomenon and b) partially explains the phenomenon of “muscle memory.”
Prefer to listen? Check out the audio roundtable
Every article we review in MASS has an accompanying audio roundtable just like this one. All of the MASS reviewers (Greg Nuckols, Eric Trexler, Eric Helms, and Mike Zourdos) get together to discuss the findings and applications in practical, easy-to-understand terms. Subscribe to MASS here.
Regarding myonuclear permanence, I think there’s sufficient evidence to claim that it is, at minimum, too simplistic. On one hand, the absolute highest quality studies we have (utilizing longitudinal in vivo imaging) all report that myonuclei aren’t lost following atrophy in rodent models. On the other hand, half of the animal studies using the second best method for studying myonuclei (staining myonuclei, cell membranes, and satellite cells) report a decrease in myonuclei following atrophy, and more than half of the cross-sectional human research is consistent with a loss in myonuclei during age-related atrophy. “Myonuclear permanence” implies that all myonuclei stick around forever, but my interpretation of these findings is that at least some myonuclei can be lost during or following significant muscle atrophy.
However, “myonuclei can be lost during or following atrophy” is not necessarily synonymous with “myonuclei are lost at the same rate that fibers atrophy.” I think one of two potential explanations (or a combination of both) would explain the bulk of the seemingly disparate findings:
- Myonuclei can be lost following atrophy, but the loss of myonuclei lags behind other atrophic processes to a significant degree.
- Myonuclei are not lost following atrophy in young people, but can be lost following atrophy in older adults
To unpack the first potential explanation, let’s assume someone’s muscle fibers decrease in size by 20% following six months of detraining. It may be the case that the loss of myonuclei lags by a few months, or even a few years. After those same six months of detraining, all of the myonuclei may still be sticking around, meaning that the myonuclear domain size has decreased. However, after a year of detraining, maybe myonuclear content has decreased by 10%, and after two years, myonuclear content has decreased 20%, matching the loss of total fiber size. Under this model, myonuclei aren’t permanent, but perhaps they could assist somewhat with “muscle memory” if the detraining period is less than two years long. To be clear, I’m not proposing that this is the actual time course of myonuclei loss; these time increments are just being used to illustrate the model.
The second explanation is more straightforward – myonuclei are more likely to be lost as someone gets older. Maybe you work out in high school and college, build a lot of muscle, accrue a lot of myonuclei, and then stop training for a decade. You lose all the muscle you built, but you retain all of the myonuclei you gained, helping you rebuild size and strength when you get back in the gym in your 30s. However, you may start losing those myonuclei in your 50s, and have myonuclear content matching your muscle fiber size (i.e. have the same myonuclear domain size as someone who’d never worked out) by the time you turn 70. Again, these numbers are for illustrative purposes only.
A blend of these two models might look something like this: You work out in high school and college, build a lot of muscle, accrue a lot of myonuclei, and then stop training. Over the next decade, you may lose myonuclei following muscle atrophy, but the process is slow. If your myonuclear content increased by 20% when you were training, maybe it’s still elevated by 10% if you take 10 years off of training in your 20s or 30s. However, if you stopped training at 70 years old instead of 22 years old, you may lose myonuclei at a much faster rate. If it takes you six months to lose all the muscle you built, maybe it only takes a year for all of those new myonuclei to be lost as well.
I’m not sure which of these three models is the closest to being completely correct, but I think all three are closer to the “truth” than either simplistic model – myonuclear permanence, or myonuclear apoptosis occurring at the same rate as muscle fiber atrophy.
The next obvious question is, “if those myonuclei do stick around for a pretty long time, does that actually matter?” Remember, one idea underpinning the myonuclei-mediated muscle memory concept is the idea that greater myonuclear density (smaller myonuclear domains) is equated with enhanced transcriptional capacity, leading to more muscle proteins being built following a resistance training stimulus. As mentioned previously, two studies have examined this idea (7, 8). A study with young subjects found that people with initially smaller myonuclear domains did not build muscle faster (7), while a study with older subjects found that subjects with initially smaller myonuclear domains were able to build muscle faster (8). The difference seems to relate to the subjects’ ability to accrue more myonuclei. In the young subjects, folks with larger myonuclear domains just accrued more myonuclei and grew without a hitch. In the older subjects, new myonuclei were harder to gain, so a smaller initial myonuclear domain was beneficial. This leads me to believe that, all else being equal, this piece of the myonuclei-related muscle memory model makes some sense – if your muscles atrophy but you retain your myonuclei, that boost in transcriptional capacity might increase muscle growth to some degree. However, once you add in one more variable – the ability to easily accrue more myonuclei (as was seen in the study on younger subjects) – it doesn’t seem that smaller myonuclear domains matter much. Thus, mechanistically, the “muscle memory” effects of myonuclei may actually be pretty small for most people. They may play a bigger role in people who have a harder time accruing more myonuclei (like elderly people and folks who struggle with muscle growth generally), but if you’re someone who’s relatively young and if you’ve previously had decent success with building muscle, having more myonuclei sticking around after a layoff may not actually contribute a huge amount to the “muscle memory” response you see when you start training again.
As a matter of fact, after re-reading the seminal Egner study (2) demonstrating muscle memory in mice with a more critical eye, it became clear that the myonuclei likely couldn’t fully explain the “muscle memory” response in that study. Following the three weeks of muscle atrophy in the group of mice that previously received testosterone, their muscle fibers were the same size as the mice that had received a sham treatment, and their myonuclear density (myonuclei per mm of fiber length) was approximately 15% greater. Following two weeks of overload, fiber growth was roughly two-fold greater in the mice that had previously received testosterone. Just using rough numbers, if a 15% greater myonuclear density can increase transcriptional capacity by 15%, but you’re attempting to explain a 100% difference in hypertrophy, some other mechanism(s) must be accounting for the remaining 85% difference in hypertrophy. Thus, retaining myonuclei following atrophy may be a mechanism of “muscle memory,” but it can’t be the only mechanism, and it’s likely not the most important mechanism.
However, I do think there are two instances where maintenance of myonuclei following atrophy could still be relevant. The first is aging. Since elderly people have a harder time accruing new myonuclei, if we found that elderly people have smaller myonuclear domains if they’d previously lifted weights when they were younger, that would give us another benefit to list when promoting resistance training. The second is athletics. If steroids allow athletes to accrue WAY more myonuclei than they’d be able to accrue naturally, those athletes may wind up with a very long-lasting advantage over lifetime drug-free athletes. For example, if steroids help someone build 50% more muscle than they’d have been able to build drug-free, and most of those myonuclei stick around after they stop using steroids, they may be able to maintain enough enough of those gains that they wind up with 20% more muscle than they’d have been able to build drug-free. If those myonuclei all stick around for, say, 10 years (or longer), it would make the current WADA doping bans (2 years for a first offense) seem woefully inadequate, opening up the discussion of lifetime bans for athletes caught using anabolic steroids.
So, if myonuclei can’t fully explain the muscle memory phenomenon, what are some other possible mechanisms?
One leading candidate is epigenetic modification (10). We’ve written about that previously in MASS. I’m honestly not sure about other mechanisms, because muscle tissue is highly plastic, meaning it has the capacity to change and adapt rapidly (relative to most other tissues, at least), but mechanisms of muscle memory would need to involve changes that persist for at least months, if not years. Proteins are replaced regularly, mitochondrial density decreases rapidly following detraining, capillarization decreases within a few weeks, etc. One possibility is ribosome density. Ribosomal content may be associated with hypertrophy (11), and I honestly have no idea how quickly ribosomal content drops following detraining. Another possibility could be a neural mechanism of some sort. Instead of needing to completely relearn motor patterns, you still retain some of your motor skills following a layoff, perhaps allowing you to simply present your muscles with a greater stimulus than a brand new lifter would be capable of inducing. Muscle memory is an interesting concept, and I believe the current research in the area is barely scratching the surface.
Next Steps
We need some longitudinal studies in humans. A study designed like the Psilander study, but that actually induced enough hypertrophy to cause myonuclear accretion would be a good start. For cross-sectional studies, we need some studies with intermediate ages included. Most of the research is looking for differences between 25 year olds and 70 year olds, but those studies can’t tell us anything about the time course of age-related myonuclear adaptations. Including a cohort of 40-50 year olds would help considerably. I’d also be interested in a longitudinal study simply cataloging the rate of myonuclei loss in humans following 5-10 years of detraining. Have a group of college students train for 6-12 months (long enough to build a fair amount of muscle and accrue a fair amount of myonuclei), and take biopsies periodically to observe how myonuclear content changes over a long period of time. Finally, I’d love to see a detraining study on athletes who stop taking steroids, but keep training hard. How much muscle do they end up losing? How does their myonuclear content compare to people who’ve never used steroids? Do they lose myonuclei when they stop using steroids?
Application and Takeaways
The role of myonuclei in muscle memory is … hazy. It does seem that myonuclei are lost at a slower rate than muscle fibers atrophy, but your myonuclei probably aren’t actually permanent. Myonuclei may play a role in “muscle memory,” but other mechanisms likely explain the lion’s share of the phenomenon.
Subscribe to MASS Research Review
This article was first published in MASS Research Review.
With MASS, you'll get concise and applicable breakdowns of the latest strength, physique, and nutrition research – delivered monthly.
Learn more and subscribe

Share this on Facebook and join in the conversation
References
- Snijders T, Aussieker T, Holwerda A, Parise G, van Loon LJC, Verdijk LB. The concept of skeletal muscle memory: Evidence from animal and human studies. Acta Physiol (Oxf). 2020;229(3):e13465. doi:10.1111/apha.13465
- Egner IM, Bruusgaard JC, Eftestøl E, Gundersen K. A cellular memory mechanism aids overload hypertrophy in muscle long after an episodic exposure to anabolic steroids. J Physiol. 2013;591(24):6221-6230. doi:10.1113/jphysiol.2013.264457
- Gundersen K. Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J Exp Biol. 2016;219(Pt 2):235-242. doi:10.1242/jeb.124495
- Bruusgaard JC, Egner IM, Larsen TK, Dupre-Aucouturier S, Desplanches D, Gundersen K. No change in myonuclear number during muscle unloading and reloading. J Appl Physiol (1985). 2012;113(2):290-296. doi:10.1152/japplphysiol.00436.2012
- Psilander N, Eftestøl E, Cumming KT, et al. Effects of training, detraining, and retraining on strength, hypertrophy, and myonuclear number in human skeletal muscle. J Appl Physiol (1985). 2019;126(6):1636-1645. doi:10.1152/japplphysiol.00917.2018
- Snijders T, Leenders M, de Groot LCPGM, van Loon LJC, Verdijk LB. Muscle mass and strength gains following 6 months of resistance type exercise training are only partly preserved within one year with autonomous exercise continuation in older adults. Exp Gerontol. 2019;121:71-78. doi:10.1016/j.exger.2019.04.002
- Snijders T, Smeets JS, van Kranenburg J, Kies AK, van Loon LJ, Verdijk LB. Changes in myonuclear domain size do not precede muscle hypertrophy during prolonged resistance-type exercise training. Acta Physiol (Oxf). 2016;216(2):231-239. doi:10.1111/apha.12609
- Holwerda AM, Overkamp M, Paulussen KJM, et al. Protein Supplementation after Exercise and before Sleep Does Not Further Augment Muscle Mass and Strength Gains during Resistance Exercise Training in Active Older Men. J Nutr. 2018;148(11):1723-1732. doi:10.1093/jn/nxy169
- Staron RS, Leonardi MJ, Karapondo DL, et al. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J Appl Physiol (1985). 1991;70(2):631-640. doi:10.1152/jappl.1991.70.2.631
- Seaborne RA, Strauss J, Cocks M, et al. Human Skeletal Muscle Possesses an Epigenetic Memory of Hypertrophy. Sci Rep. 2018;8(1):1898. Published 2018 Jan 30. doi:10.1038/s41598-018-20287-3
- Roberts MD, Haun CT, Mobley CB, et al. Physiological Differences Between Low Versus High Skeletal Muscle Hypertrophic Responders to Resistance Exercise Training: Current Perspectives and Future Research Directions. Front Physiol. 2018;9:834. Published 2018 Jul 4. doi:10.3389/fphys.2018.00834