In 2013, I showed up for a graduate school interview with a great deal of enthusiasm and a diverse array of potential research topics in my head. Later that year, I showed up to actually begin graduate school. I was about 40 pounds lighter, too depleted to display anything resembling enthusiasm, and my most pressing research question was now crystal clear: Why does dieting suck so much?
As you might have inferred, I did a bodybuilding competition that year. This is a unique case of weight loss, in which the changes occur fairly rapidly, and the end goal is an exceptionally low body fat level. However, the rigors and challenges of weight loss are not entirely reserved for physique athletes, and the robust physiological defenses against weight loss apply to a wide variety of dieters. I began my graduate school career by writing a review paper about the physiological hurdles we face in the weight loss process, which guided me toward a series of small studies on the matter. This three-part article discusses the problems we observed, the solutions I propose, and some suggestions to promote a smooth transition to life after weight loss.
Part 1. The Problem: Metabolic Adaptation To Weight Loss
The great irony of weight loss is that our modern problems were our ancient saviors. Think back to a much simpler time, before humanity was encumbered by desk jobs and student loan debt. Our evolutionary ancestors woke up each day for the purposes of obtaining nourishment, avoiding harm, and keeping the species viable, and that was enough for them. But this was also a time when food availability was highly uncertain. In terms of survival and reproductive success, fortune favored the individuals most able to store up energy when food was widely available, so they could rely on it when food was hard to come by. This is the general premise of the Thrifty Gene Hypothesis; while the hypothesis is an oversimplification that fails to fully describe the modern epidemics of obesity and cardiometabolic disease, it remains a basic but useful conceptualization of the modern plight of dieters. Our ability to effectively store fat allowed our ancestors to stay alive during times of famine, but it also predisposed us to store a whole bunch of unwanted energy (fat) in an era of unprecedented food availability. Metabolic adaptation is an intentionally broad term that describes the collection of responses our body has to fight back against weight loss attempts and can be viewed as the “other side” of the Thrifty Gene Hypothesis. While much attention has been dedicated to our ability to store fat during times of feast, metabolic adaptation addresses our body’s ability to use energy more economically in times of famine. With complementary systems to promote fat acquisition and oppose fat loss, we have been endowed with the gift (or curse) that made us perfectly suited to survive ancient famine and struggle with modern weight loss.
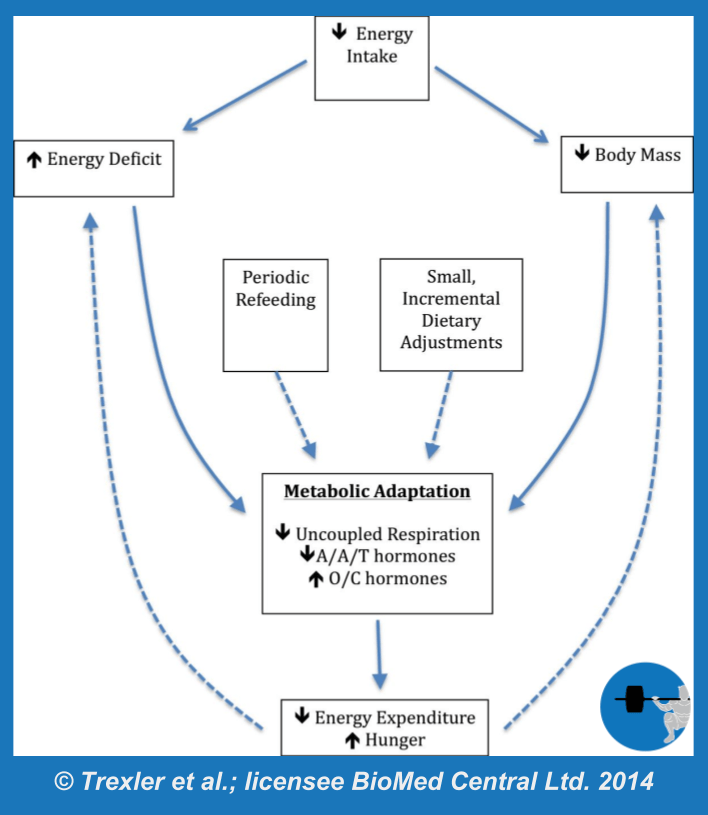
Since we’re talking about survival mechanisms, this article is written to address the challenges that come with somewhat intense weight loss attempts. A male that diets from 23% body fat to 18% body fat over a reasonable time frame is not coming face to face with his impending starvation, so the magnitude of adaptation is typically mild. When weight loss attempts become more extreme – either by losing huge amounts of weight, losing weight very rapidly, or pushing body fat storage close to the minimum amount required for survival – the adaptations become much more pronounced. While many equate metabolic adaptation (Figure 1) with an adaptive drop in energy expenditure, also known as adaptive thermogenesis, the full breadth of metabolic adaptation also involves alterations in a wide range of hormones, neuroendocrine control of appetite, and even reproductive function. The specific sites of adaptation range from the most powerful control centers of the brain to the microscopic mitochondria within our cells.
Mitochondrial Efficiency
If you remember high school biology class, you might know mitochondria as the powerhouses of the cell, and adenosine triphosphate (ATP) as the energy currency of the cell. These two metaphors provide the basic foundation from which we understand macronutrient metabolism. When you want to use energy to make a biological “purchase,” such as protein synthesis, muscular movement, nerve transmission, or virtually anything else, you’re going to need to come up with the form of energy currency that is accepted. You take in dietary energy in the form of carbohydrate, fat, protein, and alcohol; this is like accepting payments in euros, Japanese yen, and Swiss francs, but living in a place where you can only use US dollars to make purchases. Macronutrient metabolism is the process by which these various currencies are converted to the currency you need (ATP), and mitochondria play a huge role in the process.
A comprehensive review of macronutrient metabolism is well beyond the scope of this article, but a very simplified explanation will suffice. When we need some ATP, we ultimately funnel the majority of our stored or eaten energy toward two primary pathways: aerobic glycolysis and the tricarboxylic acid (TCA) cycle (also known as the Krebs cycle). Both processes directly produce a little bit of ATP, but they also produce nicotinamide adenine dinucleotide (NADH) and/or flavin adenine dinucleotide (FADH2). These make their way to the inner membrane of mitochondria, where the majority of total ATP production takes place in a process known as oxidative phosphorylation. Electrons are passed from NADH and FADH2 down the electron transport chain; this process causes protons to be pumped from inside the inner membrane to the space that exists between the inner and outer membranes of mitochondria. This establishes an electrochemical gradient, and the protons “want” to get back across the inner mitochondrial membrane.
If oxidative phosphorylation was 100% efficient, all of the protons would form an orderly line in front of the ATP synthase enzyme, use this transporter to cross back over the inner mitochondrial membrane, and ATP would be created in the process. But energy conversion isn’t 100% efficient. Consider the combustion engine in your car; the chemical energy of gasoline is converted to the mechanical energy of movement, but not with perfect efficiency. The inefficiency of this conversion results in heat production, to the extent that the engine requires a cooling system to help dissipate this excess heat. The human body is no different, and heat is the byproduct of our metabolic inefficiency. Under normal conditions, oxidative phosphorylation is about 40% efficient, give or take. This means that roughly 40% of the available energy actually gets used for ATP production, with the remaining ~60% dissipated as heat. However, this efficiency can be altered.
When some of the protons in the intermembrane space circumvent ATP synthase and “leak” back across the inner membrane, ATP is not produced in the process. In this case, the body completes the preliminary steps of metabolizing the macronutrient, releasing heat energy in the process, but doesn’t actually produce the ATP that makes the whole process worth it. This leakage is comprised of basal proton leak, which is largely dictated by a transport protein called adenine nucleotide translocase (ANT) and the fatty acyl composition of the inner mitochondrial membrane, and inducible proton leak, which is actively regulated by uncoupling proteins (UCPs). These proteins get their name from the term uncoupled respiration, which describes the process in which energy substrates are metabolized but result in heat production rather than ATP synthesis. The infamous weight loss drug 2,4-dinitrophenol (DNP) works by uncoupling respiration; the result is a potentially disastrous and sometimes fatal increase in metabolic rate and heat production. That is, of course, an extreme example that doesn’t occur with normal physiological processes.
In mammals, uncoupled respiration due to proton leak is a non-negligible contributor to energy expenditure, accounting for about 20-30% of resting metabolic rate in rats under normal circumstances. Proton leak is higher in warm-blooded animals than cold-blooded animals, and proton leak in mammals is inversely related to body mass due to differences in body temperature regulation. So, proton leak probably accounts for a smaller percentage of metabolic rate in humans compared to rats. Nonetheless, its impact is large enough to have meaningful effects, as demonstrated by the observation that proton leak correlates with weight loss success and that diet-resistant individuals with below-average weight loss outcomes tend to experience less proton leak. Aside from these apparently innate differences in proton leak between individuals, there is evidence to suggest that the magnitude of proton leak adapts in response to energy restriction and weight loss.
Much of the research pertaining to weight loss and proton leak is done in rodents; while this requires a small leap of faith in assuming that the results are relevant to human beings, it allows for an otherwise impossible level of experimental control. In rodents, studies using short-term (two weeks to two months), medium-term (six months), and long-term (12 to 18 months) calorie restriction have reported decreased proton leak, which is indicative of an adaptive increase in mitochondrial efficiency. Conceptually, it appears that mitochondria sense the relative lack of energy availability and adapt in a manner that reduces wasteful proton leak. This increases the ability of mitochondria to meet their ATP-production “quota” while burning fewer calories in the process. Exactly how they go about doing this is a bit less straightforward. In these rodent studies, an unexpected but consistent observation was that UCP-3 protein content increased, which is counterintuitive; while proton leak decreased, this protein that facilitates proton leakage actually increased. Human studies, however, indicate the opposite.
One study evaluated UCP-2 and UCP-3 mRNA levels in lean, obese, and weight-reduced humans. They found that muscle levels of UCP-3 were reduced in people who had lost weight and stabilized at their reduced weight, which is intuitively consistent with reduced proton leakage. Another study by a different research group reported similar findings, with lower UCP-3 mRNA expression observed in individuals who had undergone prolonged energy restriction to induce weight loss. Perhaps the strongest evidence supporting the importance of proton leak and UCP-3 comes from a 2002 study in which “diet-responsive” and “diet-resistant” individuals were classified based on weight loss results, then compared on a variety of parameters. Results showed that diet-responsive individuals lost 43% more weight, and this difference was accompanied by significantly higher rates of proton leak and UCP-3 mRNA levels. Taken together, it seems likely that rodents and humans share a common strategy in which mitochondrial proton leakage is reduced in response to energy restriction, as a fairly straightforward means of conserving scarce energy stores. While differing changes in UCP-3 expression between rodents and humans are less straightforward, it is possible that subtle distinctions exist with regard to the mechanisms by which they restrict proton leakage.
The Role of Hormones
Uncoupled respiration and proton leak are heavily mediated by hormonal controls. One of the most notable hormones affecting energy expenditure is thyroid hormone (TH; particularly the more biologically active form, triiodothyronine [T3]). Low TH levels are consistently associated with low metabolic rates, and high TH levels are consistently associated with high metabolic rates. Part of this effect is related to the fact that TH stimulates ATP-consuming processes and muscle ATP consumption, which increases the body’s overall demand for ATP. When ATP demand is increased, production increases to match, which requires us to burn more of the calories we consume and/or tap into our fat storage for energy. A substantial portion of TH’s effect on metabolic rate, however, is related to proton leak. Thyroid hormone increases basal leak, with protons simply traversing the inner membrane itself or being transported via ANT, and also increases inducible leakage by stimulating UCP-3 expression and activation. In a large number of weight loss studies, including both case studies and observational studies in physique athletes, an unfortunate reality of weight loss is a reduction in TH levels, which likely contributes to a reduction in proton leak.
Thyroid hormone also stimulates the metabolic activity of brown adipose tissue (BAT), a type of fat tissue that specializes in uncoupled respiration. The primary purpose of BAT is to generate heat. Its mitochondria are loaded with uncoupling proteins; when BAT is activated, it burns calories like crazy, but yields a bunch of heat rather than ATP due to this uncoupling. While BAT makes substantial contributions to energy expenditure in small mammals and human babies, especially in cold conditions, it was long thought that humans do not have BAT in adulthood. Advances in research techniques have increasingly enabled researchers to identify the presence of brown adipose tissue (and a similar thermogenic fat known as beige adipose tissue) in adults in amounts that could make small, but non-negligible, contributions to energy expenditure. While BAT is likely to play a very small role in the overall picture of energy expenditure during weight loss, reduced activation of this tissue via TH reductions may contribute to weight loss-induced reductions in uncoupled respiration.
When it comes to metabolic adaptation, TH is definitely not the hormone assuming most of the blame. In fact, the observed drop in TH is influenced, at least in part, by a more influential hormone called leptin. Leptin is primarily produced by fat cells, but production is only high when they are full and happy. When we lose weight, our fat cells store less energy than they used to, and they begin producing less leptin as they shrink in size. Even before we lose a meaningful amount of weight, leptin drops substantially just from a few days of energy restriction, which is most closely related to the reduction of dietary carbohydrate (rather than fat or protein). The hypothalamus is a brain structure that serves as a key regulator of appetite and metabolism, and leptin receptors in the hypothalamus allow fat cells to communicate their fullness directly to the brain. As we’ll see throughout this article, leptin is a high-profile mediator of countless aspects of metabolic adaptation (Figure 2), eliciting effects on TH, reproductive hormones, hunger hormones, cortisol, and multiple components of energy expenditure. Metabolic adaptation entails a lot more than just reduced energy expenditure, and the widespread effects of leptin are a major reason for that. Staying on the topic of uncoupled respiration and BAT, high leptin levels stimulate the sympathetic nervous system via the hypothalamus, which results in activation of BAT and a resulting increase in energy expenditure. Leptin also stimulates thermogenesis (i.e., energy expenditure) directly in skeletal muscle, independently of BAT. Weight loss studies consistently show reduced leptin levels, with a corresponding reduction in energy expenditure. In addition, human BAT is indirectly activated by insulin as well. This insulin-mediated effect is most markedly promoted by high-carbohydrate meals, which, unfortunately, tend to be pretty hard to come by in the later stages of a weight loss diet.
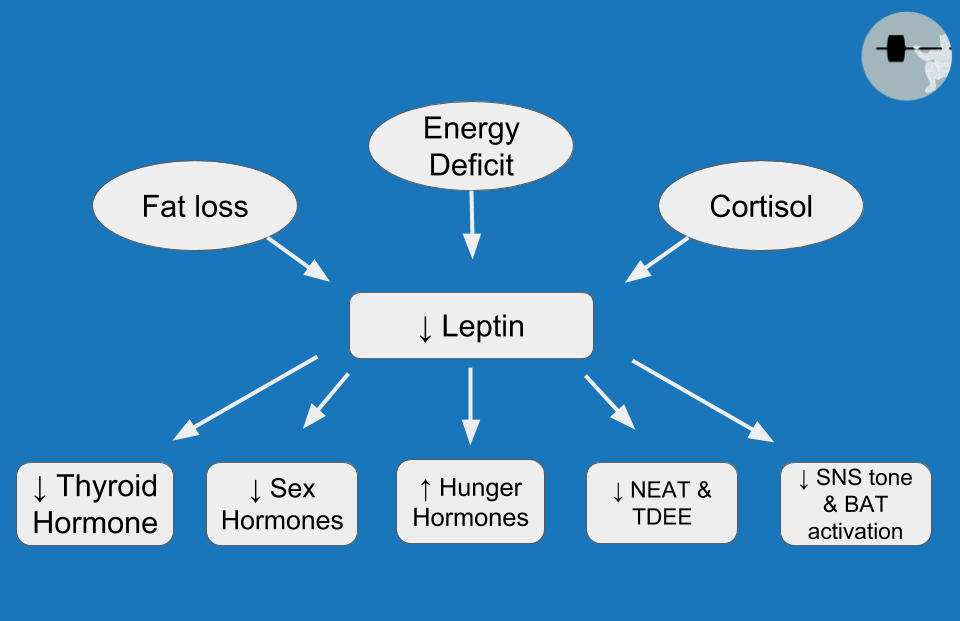
There are also hormonal effects of energy restriction that extend beyond mitochondrial efficiency or adaptive thermogenesis. Several case studies have been carried out in physique athletes, and alterations in anabolic and catabolic hormones are pronounced and consistent. One such case study documented reductions in testosterone while cortisol increased; our group did a similar case study and found similar changes in testosterone and cortisol. Recently, there has been a lot of discussion about whether or not post-exercise changes in testosterone play an important role in muscle growth, and the common consensus is that they do not. But it’s critically important to distinguish normal, resting testosterone levels from short-term, post-exercise elevations. Resting testosterone levels do appear to have an impact on the growth and maintenance of lean mass (otherwise, professional athletes probably wouldn’t have consistently risked their careers to elevate it for the last several decades). Aside from its impact on BAT activation, insulin is also known to have anabolic properties. While there is some debate over how significant this effect is within normal physiological ranges of insulin, a study in bodybuilders documented a reduction in fasting insulin levels during contest preparation, and insulin changes were significantly correlated with changes in lean mass. This means that people who had larger insulin reductions tended to lose the most lean mass. However, it’s important to note that they also tended to lose more fat mass, so this could potentially just be demonstrating that some individuals preserved some additional lean mass by failing to get super lean.
Increased cortisol also contributes to the threat of lean mass loss while dieting. Multiple case studies have reported elevated cortisol late in contest preparation, which likely relates to the combination of energy restriction, training load, and the general stress that accompanies competition. Manipulating cortisol within normal physiological ranges has the capacity to alter the maintenance of body proteins, with higher levels inducing protein breakdown. As we might expect, there are a couple of case studies showing pretty substantial loss of lean mass (more than 10 pounds of it) throughout contest preparation in male bodybuilders. Taken together, these collective hormonal disruptions during intensive weight loss threaten the ability to maintain lean mass throughout the diet. This is problematic for multiple reasons: the loss of lean mass directly opposes the goals of just about any athlete attempting to lose weight, lean mass is the primary determinant of resting metabolic rate, and such a loss of lean mass is also linked to excessive hunger until that lean mass is restored.
To make matters worse, loss of lean mass is not the only thing driving excessive hunger. Hunger is probably the most undesirable side effect of dieting for fat loss, and there are multiple hormonal responses that play a direct role in the urge to eat. Ghrelin is mainly produced in the stomach and is primarily known as a messenger to keep the brain informed about short-term energy availability. After a meal, the stomach produces less ghrelin, and the brain is aware that additional energy is not acutely necessary. During times of fasting, its production increases, and levels ultimately get high enough to induce hunger. However, there is also evidence that ghrelin plays a role in sensing long-term energy availability, and diet-induced weight loss increases 24-hour ghrelin levels. Unsurprisingly, two separate case studies have documented increased ghrelin levels from the beginning to the end of contest prep in male bodybuilders.
We previously discussed leptin as a stimulator of BAT activity and muscle thermogenesis, but its hypothalamic duties do not end there. When leptin binds with its receptor in the hypothalamus, it initiates a domino-effect of signaling events that result in decreased neuropeptide Y (NPY) and agouti-related peptide (AgRP), with increased pro-opiomelanocortin (POMC) and cocaine-and-amphetamine-regulated transcript (CART). The result is that leptin stimulates energy expenditure while also reducing hunger. A weight loss-induced drop in leptin therefore has the unpleasant bonus of reducing the number of calories you burn, while increasing your desire to consume more of them. We know insulin as a potentially anabolic hormone associated with lean mass retention, but it has appetite-related roles as well. Insulin receptors are present throughout multiple brain structures, including the hypothalamus, and insulin reduces appetite upon binding to these receptors. Cortisol comes into play here as well, as glucocorticoids (such as cortisol) oppose the effects of leptin. It’s problematic enough that leptin is reduced during energy restriction and weight loss, but increases in cortisol also impair the biological function of the leptin that is still around, thereby amplifying the negative effects of low leptin levels. Cortisol also promotes fluid retention, at least in part by binding to mineralocorticoid receptors, and is linked with appetite stimulation and increased cravings for palatable foods. Collectively, chronic elevation of cortisol can promote loss of lean tissue, make weight loss appear to stall by increasing water retention, exacerbate the effects of low leptin levels, and potentially increase urges to overeat. As weight loss causes increases in ghrelin and cortisol with reductions in leptin and insulin, this four-pronged attack on your hunger regulation yields a very unhappy dieter.
Hormonal adaptations to weight loss also have the capacity to interfere with reproductive function, thereby amplifying the unhappiness. Intuitively, this makes a great deal of sense from an evolutionary perspective. Low energy availability is perceived as a sign of insufficient resources in the environment, which is only exacerbated by the energy cost of gestation and further division of resources to accommodate a population increase via reproduction. During contest preparation, male testosterone levels don’t just drop, they plummet. Aside from the obvious threat to muscle mass retention, lack of libido is a very common complaint of male physique athletes late in the contest preparation process. Similarly, female athletes typically experience a drop in estradiol in response to contest prep, along with menstrual cycle disruption. The severity of this disruption may depend upon a variety of factors, including acute energy availability, magnitude of overall weight loss, body fat percentage, and genetic predisposition. In less severe cases, menstruation may simply become more irregular; in more severe cases, menstruation may cease altogether. A case study of a female figure competitor revealed that menstruation became disrupted early in the contest preparation process, had ceased by the end of prep, and did not return until a full 71 weeks after competition. These reproductive side effects are not to be taken lightly; in addition to being generally unpleasant, there are long-term consequences to maintaining a prolonged state of sex hormone suppression, such as impaired bone health and fertility. The primary hormonal adaptations to weight loss are summarized in Figure 3.
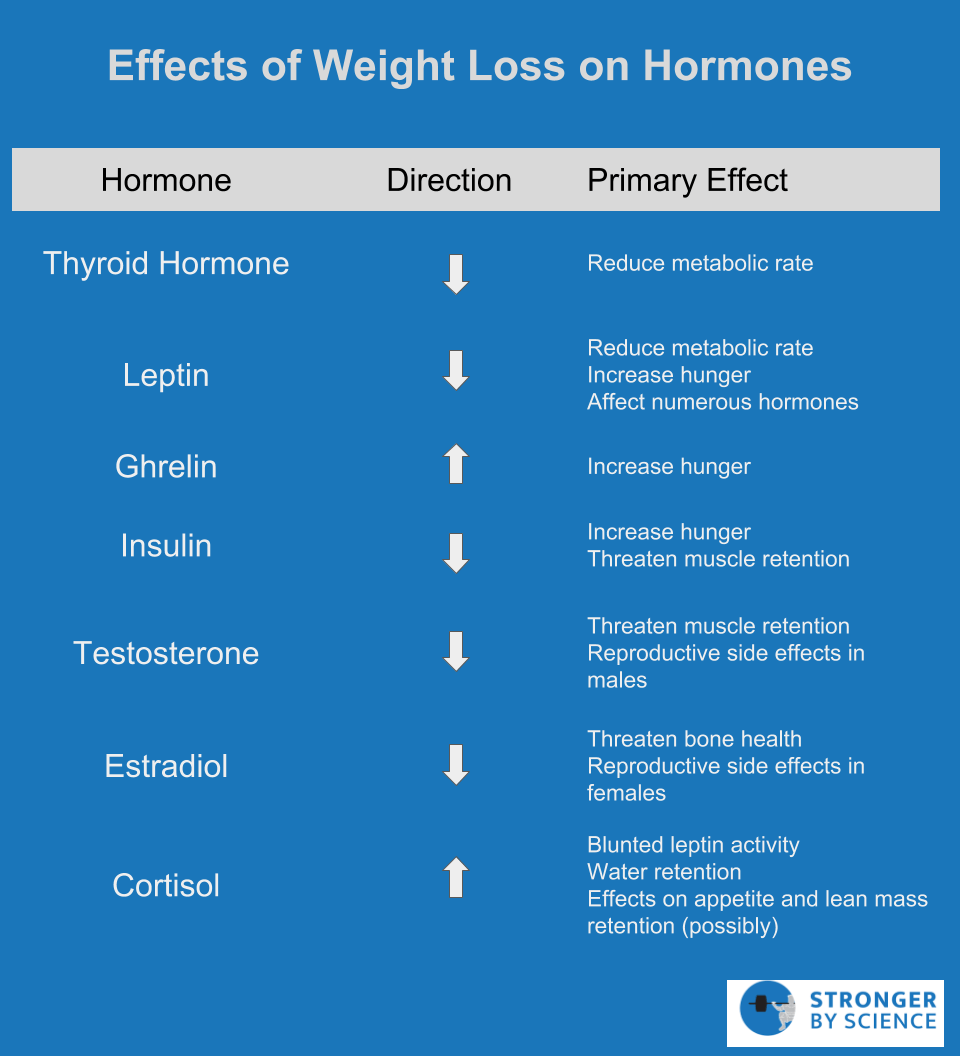
As the previous sections would suggest, I prefer not to think of metabolic adaptation as just a drop in metabolic rate. Rather, I prefer to conceptualize it as a multifaceted set of adaptations that involves regulation of energy expenditure, appetite, reproductive function, and the balance between anabolism and catabolism. In recent years, the International Olympic Committee has used the term “relative energy deficiency in sport,” or “RED-S,” to summarize a similar physiological state. The biological goal is to constrain energy use during times of underfeeding, but the effects are widespread, and to discuss them in isolation from one another is a bit short-sighted. Despite this wide variety of adaptations to weight loss and energy deficiency, the drop in energy expenditure, also known as adaptive thermogenesis, is the one that garners much of the attention in the fitness world. Up to this point, we’ve mentioned energy expenditure in a couple of esoteric contexts that have yet to be linked together in a cohesive manner. We have talked about proton leak, brown adipose tissue activation, and hormones related to ATP consumption and thermogenesis. But physique athletes and other dieters rarely utter a single one of those words in day-to-day conversation about dieting. So, it’s time to put some of these concepts together to discuss the aspect of metabolic adaptation that everyone cares about: energy expenditure.
Effects on Energy Expenditure
The effects of metabolic adaptation on energy expenditure are the ones everyone cares about, because they’re the ones we actually tend to act on by manipulating our diet and exercise protocols. It becomes apparent that our energy expenditure is lower when our weight loss slows down, so we have to either reduce food intake or increase cardio, and we’d much prefer to do neither. But before getting into specific aspects of metabolic rate adaptations, we need a quick primer on energy expenditure in general.
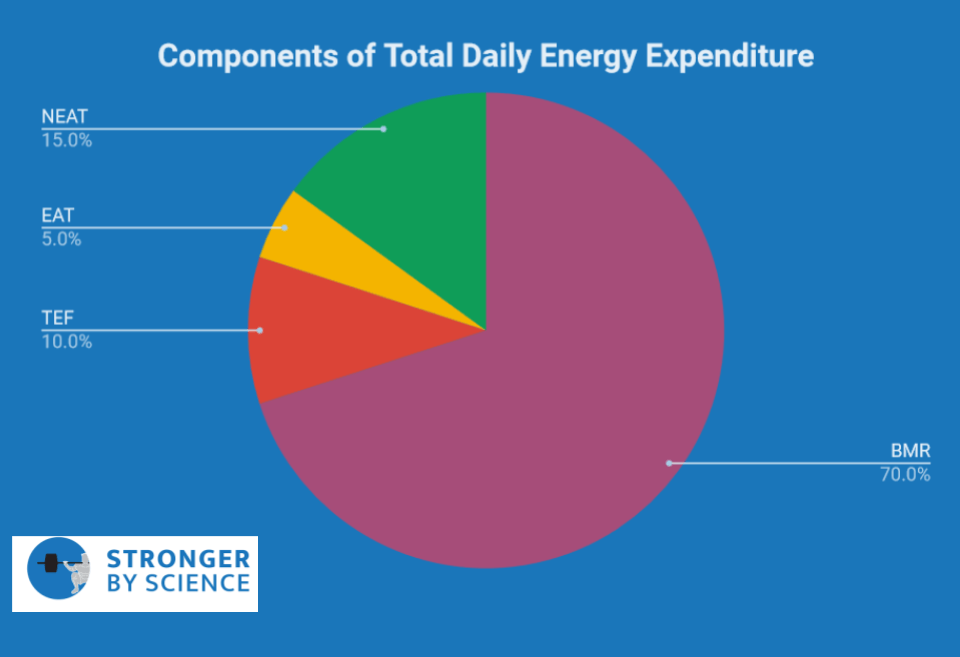
Every day we consume energy and we burn energy; the net balance of this equation determines whether we gain or lose weight. As indicated in Figure 4, total daily energy expenditure (TDEE) describes the total number of calories we burn in a given day, and TDEE is made up of four components:
- Basal Metabolic Rate (~70% of TDEE in general population)
- This describes the energy required to simply keep our body “on,” at rest, assuming we lay in bed all day without moving or eating.
- Thermic effect of feeding (~10% of TDEE in general population)
- This describes the energy used in the process of eating, digesting, metabolizing, and storing food.
- Exercise Activity Thermogenesis (~5% of TDEE, depending on how much you exercise)
- This describes the energy used during structured, intentional exercise.
- Non-Exercise Activity Thermogenesis (~15% of TDEE, depending on your activity level)
- This describes the energy used for any movement that isn’t purposeful exercise. This would include walking around your school or office, doing yard work, taking out the trash, and even fidgeting in your chair
To tie up some loose ends, let’s consider a few terms we have mentioned haven’t yet fully contextualized. We know that brown adipose tissue increases thermogenesis, particularly in response to cold exposure, thyroid hormone, leptin, and insulin; BAT thermogenesis would therefore primarily affect basal metabolic rate and the thermic effect of feeding. We noted that thyroid hormone and leptin increase mitochondrial uncoupling and muscle thermogenesis; these broad effects would have the potential to affect essentially all components of energy expenditure. So, all of the mitochondrial and thermogenesis-related concepts previously discussed fit somewhere within our four components of energy expenditure, and sometimes span multiple components. Unfortunately, when we embark on a weight loss diet, all four components are influenced to some extent.
Thermic Effect of Feeding
In weight loss diets, the thermic effect of feeding will be reduced for a very straightforward reason, with no need for any biologically adaptive explanation: If you eat less food, you won’t burn as many calories in the process of eating, digesting, metabolizing, and storing it. It remains unclear whether or not we have an adaptive shift in the thermic effect of feeding (that is, a disproportionate increase in the efficiency with which we handle the energy cost of feeding). While there are some isolated studies suggesting that a small adaptation favoring a relatively lower thermic effect of feeding does occur, the collective evidence would suggest that any such adaptation is negligible in magnitude. For example, Miles et al found that feeding caused subjects to burn 13% of the calories consumed in an experimental meal before weight loss. After losing ~7.3 kg over a 12-14 week period, participants burned 12.5% of the calories consumed in the very same meal; this amounts to a difference of 3 Calories. Leibel et al carried out an ambitious study in which some subjects were over-fed to induce weight gain, and others were under-fed to induce weight loss. Their results showed that weight gain from intentional overfeeding did reduce the energy efficiency of feeding, leading to a relatively greater thermic effect of feeding. In contrast, weight loss from intentional under-feeding did not increase this efficiency, and the thermic effect of feeding was not significantly different when comparing baseline values to the values obtained after weight loss. Overall, the body of evidence suggests that the thermic effect of feeding is lower during weight loss dieting, but this is caused by lower overall food intake and less sympathetic nervous system activation in response to caloric intake. If there is any adaptation that makes us more efficient with regard to this thermic effect, it is very small in magnitude.
Basal Metabolic Rate
A true “basal” metabolic rate is difficult to measure. It requires an overnight laboratory visit to allow for metabolic rate assessment directly upon waking, in the absence of food intake or substantial movement. As a result, labs that are not equipped to house overnight guests often use resting metabolic rate as a surrogate measurement. They still require subjects to fast overnight, but they let the subject come to the lab in the morning. To account for the movement associated with transportation to the lab, they have the subjects lay down and rest for a while before the actual measurement occurs. Under these conditions, resting metabolic rate can be used as a pretty solid representation of basal metabolic rate, and the terms are often used fairly interchangeably in the literature.
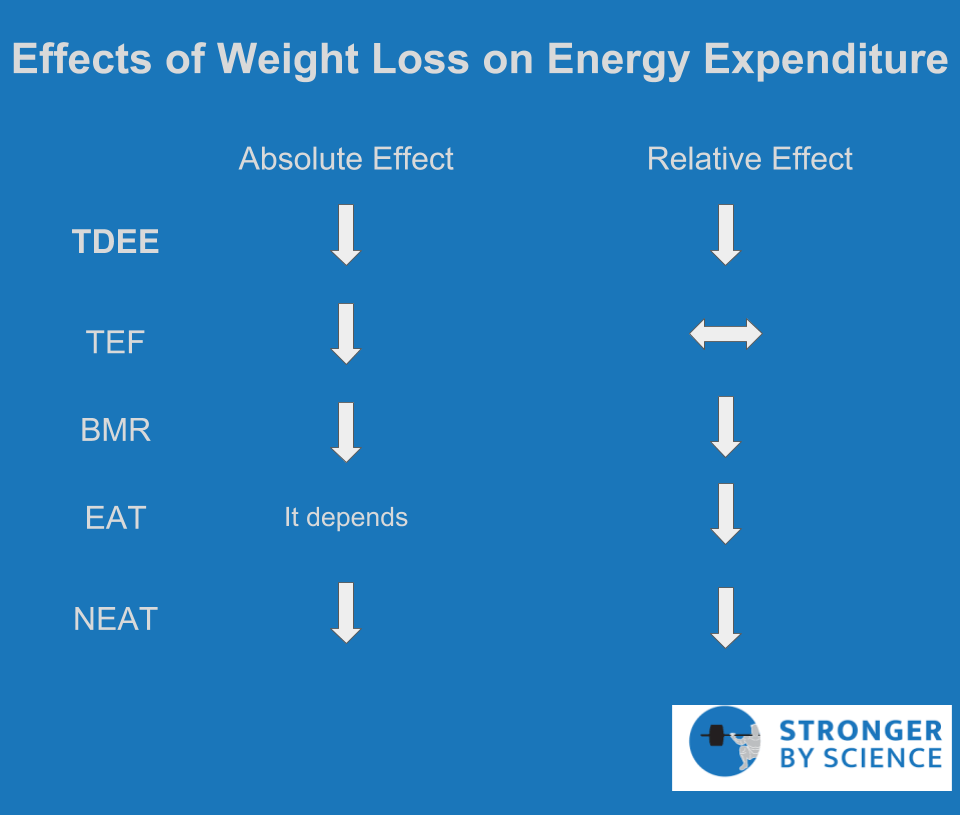
Weight loss typically involves the loss of both fat mass and fat-free mass; fat mass is a metabolically active tissue, and fat-free mass is the primary determinant of energy expenditure at rest. As a result, we fully expect resting metabolic rate to drop as we lose weight. In many cases, the drop in resting energy expenditure is greater than we would expect based purely on the loss of tissue, which suggests that an energy-conserving adaptation has taken place. The study by Leibel et al that evaluated the thermic effect of feeding also evaluated resting energy expenditure; their results indicated that resting metabolic rate was reduced following weight loss, even when accounting for the amount of fat-free mass lost. While there are some studies showing no effect of weight loss on resting metabolic rate, a meta-analysis on the topic supports the findings of Leibel et al and suggests that resting metabolic rate is indeed slightly lower in weight-reduced individuals compared to controls. These data match up quite favorably with data obtained from competitive physique athletes. For example, case studies have reported drops in resting metabolic rate that far exceed a magnitude attributable to lean mass loss and have shown resting metabolic rates to be consistently lower than predicted, even after accounting for the amount of lean mass. These low values in physique athletes are almost certainly related to the acute effects of energy restriction at the time of measurement, but the loss of fat mass is most likely a contributing factor. Collectively, the evidence would suggest that weight loss typically induces a small (but not negligible) adaptive effect that reduces resting energy expenditure, and that this is observed in both non-athletic populations and competitive physique athletes.
Exercise Activity Thermogenesis
Exercise activity thermogenesis is a tough one, because we directly control it. You decide how much weight training and cardio to do, so you have the capacity to influence it substantially. For the sake of this article, we’ll just consider the relative energy efficiency of exercise, which describes how much energy you burn for a fixed amount of exercise.
There are a couple “non-metabolic” reasons why you could use less energy for the same amount of cardio as your weight loss diet progresses forward. For any kind of locomotive cardio, such as walking, running, or cycling, the task itself involves generating enough force to transport the mass of your body. As you lose weight, the mass is reduced, and you naturally require less energy to transport your body a given distance. Even for some non-locomotive tasks like stationary cycling, weight of the limbs is reduced, so slightly less energy is required to perform the same amount of cycling work. Another factor that is rarely discussed relates to the fact that a lot of people, myself included, do absolutely no cardio unless required to. Many forms of cardio are trainable skills that we become substantially more proficient at as we do them more frequently throughout a weight loss attempt. Using running as an example, there are about 22 factors (give or take) that relate to running economy. Many of them are trainable, meaning that a novice runner can probably make substantial improvements to their running economy over the course of a few months. While this improvement in running economy is great for your joints, it also means you’re probably covering the same distance with greater efficiency, and therefore burning fewer calories in the process. Aside from these predictable reductions in exercise activity thermogenesis in response to a weight loss attempt, there are also metabolic adaptations that increase energy efficiency and reduce energy expenditure during exercise.
Researchers at Columbia did a study in which participants completed a cycling test at their normal body weight, then repeated the test after either gaining or losing 10% of their body weight. As the researchers hypothesized, muscle work efficiency at lower exercise workloads was increased following weight loss and decreased following weight gain. This means that weight gain caused the participants to use more energy to produce the same amount of physical work, whereas weight loss caused the participants to use less energy to produce the same amount of work. Several years later, the same lab group replicated their finding, observing that muscle work efficiency was increased by weight loss and decreased by weight gain. One concern might be that the participants’ legs weighed less after weight loss, which may affect the energy expenditure of cycling. Fortunately, the researchers used little weights in one of these studies to replace the leg weight that was lost; while the magnitude of the effect was smaller after weight replacement, a significant effect remained. So, it’s likely that exercise activity thermogenesis does indeed decrease (relatively speaking) throughout the course of a contest prep, and this effect is not exclusively the result of a lighter body or technique-related improvements in exercise economy. This relative decrease can certainly be offset by increasing the absolute amount of exercise. However, as we’ll see in Part 2, such a decision is not without drawbacks.
Non-Exercise Activity Thermogenesis
Non-exercise activity thermogenesis (NEAT) is the final component to discuss, and it is unequivocally the most influential when it comes to adaptive thermogenesis. Part of its impact relates to its capacity to be so incredibly variable. If I know your body size and composition, I can give a pretty good estimate of your resting metabolic rate. If I know your macronutrient intakes, I can give a pretty good estimate of your thermic effect of feeding. But two individuals of similar body size can have a daily difference of up to 2,000 Calories when it comes to NEAT. This variability is huge but not surprising when you consider the wide range of factors that can affect NEAT. The hypothalamus plays a primary role in regulating NEAT, in conjunction with components of the hindbrain and mesolimbic system. The degree of NEAT is likely to vary based on occupation, sex, age, and season, and the exact cues that feed into these regulatory centers include temperature, activity level, food intake, body composition, and a large number of neuroendocrine inputs.
To mathematically quantify the importance of NEAT in adaptive thermogenesis, let’s use the approximate numbers presented in a review by Rosenbaum and Leibel, who are absolute legends in this area: When a person loses 10% or more of their body weight, their total daily energy expenditure drops by around 20-25%. Some of this drop relates to the loss of body mass, but there is also an adaptive component, as total daily energy expenditure ends up about 10-15% below what we would expect based on body mass alone. Of that adaptive component, up to 85-90% can be explained by alterations in non-resting energy expenditure, and NEAT is by far the biggest contributor. For an experimental example, Weigle et al studied the effects of a 23% weight loss on energy expenditure. The results indicated that total energy expenditure was about 24% lower than could be explained by the loss of fat-free mass, but resting metabolic rate was only 2-3% lower than predicted. Taken together, these observations would suggest that weight loss induced a fairly substantial reduction of energy expenditure, and this reduction was largely attributable to reductions in NEAT.
The problem with NEAT is that it can be a bit difficult to measure in an accurate and affordable manner. For example, Weigle et al made use of controlled feeding protocols, daily weight changes, and linear regression to make inferences about NEAT. One of the classic studies regarding weight loss and NEAT was not actually intended to study either topic and can be objectively categorized as a very fortuitous mistake. A small group of people were placed in a 3-acre dome, which was intended to be a completely closed, self-contained world with a variety of biomes, much like a dome we could theoretically set up on a moon or a different planet. This is more or less the exact plot of a 1996 Pauly Shore movie that received an implausibly low 4% rating on Rotten Tomatoes. You could argue that the study fared a bit better than the film, but it was not without some stumbling blocks. Most notably, the Biosphere inhabitants were supposed to grow all of their own food, but they were unable to successfully grow enough calories to support their energy needs. As a result, the study became an unintentional weight loss study that lasted a full two years.
Within the first six months, subjects lost 14% of their body weight, which was maintained until leaving the dome. A week after leaving the dome, energy expenditure was assessed via a 23-hour confinement in a respiratory chamber and compared to control subjects. Total energy expenditure was suppressed in the Biosphere residents compared to controls, and this difference persisted after adjusting for relevant characteristics (age, sex, fat mass, and fat-free mass). When in the respiratory chamber, control subjects had nearly twice as much spontaneous physical activity (fairly synonymous with NEAT) than the Biosphere residents. When total energy expenditure was adjusted for this factor, the difference between Biosphere subjects and control subjects was no longer significant, which further highlights the huge impact that NEAT has on energy expenditure during weight loss. Lending further support to these findings, Leibel et al found a significant reduction in energy expenditure that was not attributable to resting metabolic rate, the thermic effect of feeding, or the loss of lean mass following 10-20% body weight reduction. A separate study had participants either gain or lose 10% of their body weight, then maintain it. Weight loss resulted in an absolute non-resting energy expenditure reduction of 37.5%, whereas weight gain increased non-resting energy expenditure by 60.2%. Linear regression showed that the change in non-resting energy expenditure explained 78% of the observed change in total energy expenditure among both groups. As summarized in Figure 5, it is very clear that weight loss causes a disproportionate drop in total energy expenditure, and it is clear that NEAT is the primary component driving this effect.
Conclusion
The original question posed was, “Why does dieting suck so much?” It turns out, the research literature offers us a variety of answers to that question. Weight loss attempts are met with changes in mitochondrial function, hormone levels, and energy expenditure. These changes produce some unpleasant side effects, threaten our ability to gain (or even keep) muscle mass and strength, manipulate biological cues pertaining to the regulation of appetite and activity level, and require us to eat even fewer calories or incorporate even more cardio into our training program. When metabolic adaptation first became a big conversation in the fitness scene, it was treated as a controversial topic. However, the content discussed in Part 1 of this article is objectively noncontroversial. The scientific literature has rigorously and repeatedly shown downregulation of energy expenditure, and the long list of physiological changes that accompany it, in response to weight loss. The controversy comes from how some of this information has been discussed, such as implausible anecdotes of fat gain despite remarkably low caloric intake, or the use of less rigorous terms like starvation mode. Rest assured, there is not a single member of our species that can elude the inescapable grasp of starvation; our caloric needs for weight loss may fluctuate from person to person, but we all have a number. Furthermore, nobody is failing to lose fat because they are eating too few calories. Metabolic adaptation places speed bumps in our path to fat loss, no more and no less. But this raises the next important question: Can we do anything to circumvent these speed bumps?
Part 2. The Solutions to Metabolic Adaptation
As discussed in Part 1, one of the more pressing concerns we face during weight loss is a reduction in energy expenditure. Part of this is a result of reducing our body size, but part is also a disproportionately large reduction in total energy expenditure that is mediated by a variety of mechanisms involving the mitochondria, hormone levels, and the brain. We don’t really observe a relative drop in the thermic effect of feeding during weight loss, and the drop in resting metabolic rate is fairly modest in magnitude. But the more notable changes are related to non-resting energy expenditure and skeletal muscle work efficiency.
Leptin is one of many factors contributing to reductions in energy expenditure, but it is a key one. This hormone is reliably reduced during and following weight loss, and it directly communicates the fullness of adipocytes to the hypothalamus, a brain structure with huge impacts on energy expenditure, hormone levels, and hunger. Intuitively, this has led researchers to wonder if leptin replacement injections would help resolve many of the unwanted changes associated with low leptin levels. The good news is that experiments have shown this to be an effective strategy, with leptin replacement injections reversing the drops observed for total energy expenditure, non-resting energy expenditure, skeletal muscle work efficiency, thyroid hormone, and gonadotropic hormones that link leptin levels with sex hormones and reproductive function. As an added bonus, leptin administration reverses the hyperphagia, or extreme hunger, observed with weight loss. The bad news is that you can’t just walk down the street and buy some pharmaceutical-grade leptin, and even if you could, it’d be really expensive. So, for our purposes, leptin replacement is off the table, and we shift our focus toward more practical strategies.
Dealing With Reductions in Energy Expenditure
While we know that the reduction of non-exercise activity thermogenesis (NEAT) is a huge contributor to the reduction of energy expenditure observed with weight loss, there isn’t much we can consciously do about that in terms of physical activity. Of course, we could try to be mindful of non-exercise activity during weight loss in a qualitative sense. Consider a basic example: My mailbox is about 100 feet away from my front door. I get to the front door and realize I haven’t checked the mail in a couple of days. If I’m not in contest prep, I am almost certainly walking over and checking; when I’m in contest prep, it’s far less likely. When I’m not prepping, I tend to pace around the room when I do my heavy thinking. During prep, this doesn’t happen. These small, subconscious decisions accumulate throughout the day, in addition to the stuff that is virtually imperceptible, such as the energy spent maintaining posture or fidgeting in your chair. For most people, I encourage them to simply let this stuff go. Nobody wants to feel like a hamster on a wheel, constantly forcing themselves to continue some type of pointless motion. For a lot of people, the psychological burden of trying to consistently increase NEAT costs way more than the value it returns. Some people try to maintain a particular step count during a weight loss diet using a pedometer, or plan short walks throughout the day to stay active; this is a reasonable middle ground, as long as it doesn’t drive you crazy. Nonetheless, the more practical and broadly advisable strategies for fighting metabolic adaptation involve manipulating the overall rate of weight loss and using nonlinear diet strategies such as refeeds or diet breaks.
Rate of Weight Loss
If the goal is to maintain lean mass and attenuate metabolic adaptation, it is advisable to avoid excessively rapid weight loss. There are two main drivers pushing metabolic adaptation: short-term energy availability, and long-term depletion of energy stores. When actively dieting to become very lean, both stimuli are encountered; some degree of energy restriction is unavoidable for active weight loss, and depletion of fat stores is the entire goal of the process. While the endpoint of our diet (very low body fat) is going to bring its fair share of unavoidable adaptations, we can certainly exacerbate some of the short-term stimuli for metabolic adaptation by imposing an excessively large deficit to yield more rapid results. For example, Mero et al studied the effects of two rates of weight loss in recreationally trained women; while losing 1kg per week successfully yielded more weight loss than 0.5kg per week, this came with a significant reduction in total and free testosterone levels. Garthe et al studied weight loss in a group of elite athletes, with one group losing 1.4% of their body mass per week and the other losing 0.7% per week. In order to prevent total weight loss from skewing the results, the slow weight loss group dieted for about 60% longer (5.3 weeks versus 8.5 weeks). Both groups lost the same amount of total weight, but the fast weight loss group lost a small amount of lean mass, whereas the slow weight loss group experienced a significant gain of lean mass. The slow weight loss group also had more favorable increases in maximal bench press, bench pull, squat, and countermovement jump performance. Results of this study are in line with a 2014 review of bodybuilding contest preparation recommendations, in which weight loss rates of 0.5 to 1.0% of body mass per week are recommended. While these studies provide evidence that slower rates of weight loss are generally advisable, they assume linear calorie reduction. A different, and especially intriguing, way to alter the timeline of weight loss is to implement nonlinear approaches that transiently increase or decrease caloric intake for hours, days, or even weeks at a time.
Intermittent or Non-Linear Caloric Restriction
It has long been understood that weight loss is induced by an energy deficit, but an energy deficit doesn’t necessarily need to be applied in a linear fashion. For example, let’s say we intend to induce a daily caloric deficit of 300 Calories per day. Over a 30-day month, we intend to eat 9,000 fewer Calories than we burn. But we have to decide exactly how to allocate those calories within each day, within each week, and within the month. There are some aspects of metabolic adaptation that are driven by getting really damn lean, or reducing the relative degree of energy stored in our body. There probably isn’t much we can do about those stimuli, especially for a physique athlete attempting to approach semi-starvation levels of leanness. But dietary strategies have emerged that absolutely alter acute fluctuations in energy availability; these strategies have assumed a variety of time scales, managing the way calories are distributed on an hour-to-hour, day-to-day, or week-to-week basis. But do any of these strategies manipulate acute fluctuations in energy availability in a manner that meaningfully attenuates aspects of metabolic adaptation?
Manipulating the energy deficit within a day
Looking at the hour-to-hour time frame, there are a couple of strategies to consider: manipulation of overall meal frequency, and time-restricted feeding windows. When it comes to affecting energy expenditure, high meal frequency is on page one of the bodybuilding nutrition folklore manual. The premise is that eating causes an acute increase in energy expenditure (via the thermic effect of feeding), so frequent meals serve to stoke the metabolic furnace throughout the day, keeping metabolic rate elevated. I suppose you could also stretch this to suggest that by providing a more consistent influx of calories, starvation mode is kept at bay. However, research has consistently indicated that increased meal frequency has no meaningful impact on the thermic effect of feeding, resting metabolic rate, or total daily energy expenditure.
Another popular strategy essentially aims to accomplish the opposite; rather than eating equally spaced meals throughout the day, time-restricted feeding encourages dieters to eat all of their meals within a quite narrow time window each day, often spanning 4-8 hours (Figure 6). This strategy, sometimes called intermittent fasting within the fitness world, aims to capitalize on the purported benefits of long fasting periods while still allowing for daily food intake. When it comes to time-restricted feeding, we still have a lot to learn. Only a handful of studies have been published to date, and methodological approaches vary quite a bit. Studies by Tinsley et al and Gill et al opted not to match caloric intake between the time-restricted feeding and control groups. Their results generally suggest that time-restricted feeding windows are a viable method for indirectly reducing caloric intake; great information, but not what we are looking for.
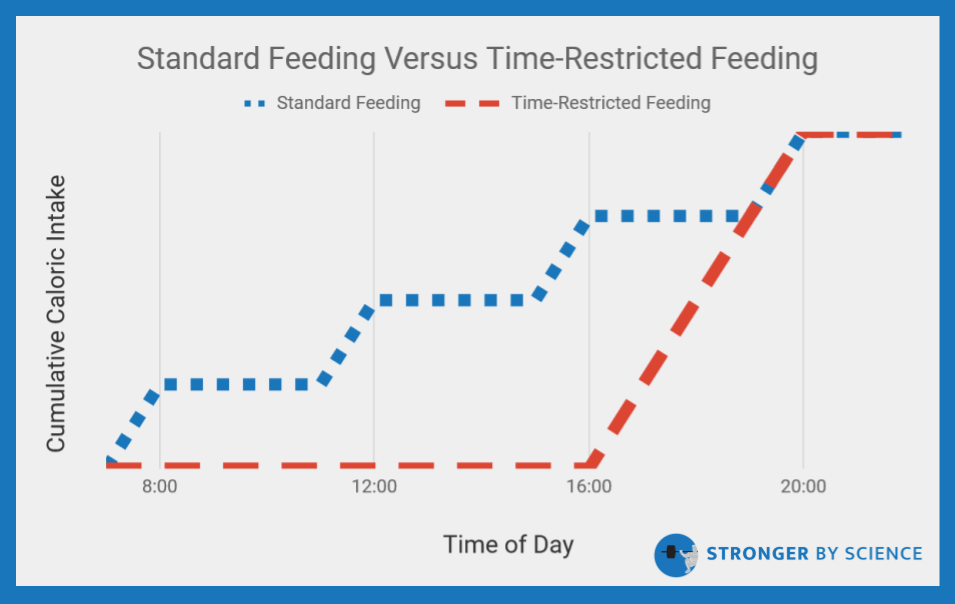
Moro et al carried out a study that attempted to match caloric intake between time-restricted and standard feeding groups during an eight-week resistance training program, but caloric intakes were set based on self-reported diet data. To their credit, they had frequent check-ins with a dietitian to monitor compliance, which is as much as you can do without actually preparing and feeding the meals to subjects. The time-restricted group lost about 1kg of weight and the control group gained about 0.2kg, but dual-energy x-ray absorptiometry results suggested a preferential fat loss in the time-restricted group (1.6kg vs. 0.3kg). Overall, it’s hard to consider this a clear “win” for time-restricted feeding. Based on very modest changes in body weight, reliance on self-reported data for setting diets, a free-living intervention without controlled meal preparation, and potential variability in responses to the resistance training program, it’s probably safest to interpret these results as time-restricted feeding being as good, with the possibility of being better, than standard feeding. Stote et al exerted a little more rigorous control over their crossover trial in which participants completed eight weeks of time-restricted feeding and eight weeks of standard feeding. They tried to match caloric intake between conditions by feeding subjects dinner in the lab and providing packed breakfast and lunch meals, but they ran into a little issue; while subjects undergoing the time-restricted feeding protocol reported higher indices of hunger on surveys taken before their daily meal, they also reported extreme fullness after the meal and struggled to finish all of the food. The subjects did lose about 1.4kg of weight over eight weeks of time-restricted feeding, but also consumed 65 fewer Calories per day compared to their standard feeding protocol. When considering day-to-day variability of weight, and this cumulative difference of more than 3600 total Calories throughout the eight-week trial, a 1.4kg difference seems like a wash.
In terms of control, a recent study conducted by Sutton et al definitely takes home the grand prize. All meals were prepared by laboratory staff and consumed in the lab, under direct supervision, utilizing a crossover design. Caloric intakes were matched over each five-week feeding period (time-restricted and standard), and the laboratory staff deliberately set caloric content of meals to prevent weight loss (they were specifically interested in looking at the effects of altering feeding windows in the absence of weight loss). They found that weight was maintained pretty effectively, with only a 0.5kg difference between the groups. The authors suggest that this difference, which favored an additional 0.5kg of weight loss in the time-restricted group, may be attributable to less glycogen storage as a result of longer fasting durations. In the other studies that attempted to match calorie intake and observed a slight fat loss benefit from time-restricted feeding, I have interpreted the favorable results with hesitation. Part of this hesitation, as previously discussed, is uncertainty over exactly how well calories were truly matched. However, my hesitation is also related to the lack of a strong mechanism that would explain any such benefit. Time-restricted feeding does not significantly affect resting energy expenditure or total daily energy expenditure, and effects have been either neutral or slightly unfavorable (from the perspective of attenuating metabolic adaptation) with regards to leptin, ghrelin, insulin, thyroid hormone, and testosterone. Stote et al suggest that it is possible that time-restricted feeding may modestly impact body composition by increasing the efflux of free fatty acids from fat cells and increasing gluconeogenesis, but this doesn’t mesh very well with the most tightly controlled study finding minimal evidence of a change in total daily energy expenditure. Moro et al propose that the modest fat mass reduction observed could be linked to increased adiponectin levels, but Stote et al exerted comparatively more dietary control and observed modest fat loss with absolutely no change in adiponectin.
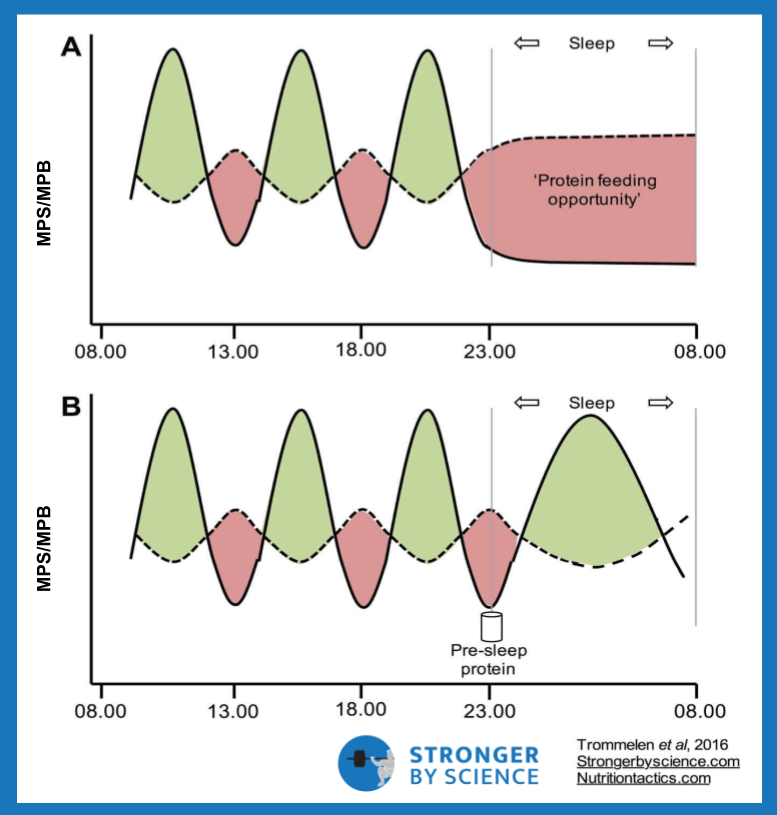
In summary, evidence suggests no benefit to increased meal frequency, at least when it comes to fat loss or energy expenditure. So, there’s no need to perpetually carry around six Tupperware containers to continuously stoke the metabolic furnace. While the time-restricted feeding data is less conclusive, it’s safe to say that time-restricted feeding has the capacity to help lower caloric intake when calories are not matched. When they are matched, time-restricted feeding is as effective as standard feeding for weight loss, with some studies suggesting a minor benefit. However, the minor benefit seems to shrink in studies that implement increasingly tighter control of energy balance, and time-restricted feeding inherently foregoes the benefits of equally spaced protein feedings on protein turnover in muscle tissue (Figure 7). To be fair, the previously cited studies that have measured lean mass do not support the idea that more lean mass is lost during time-restricted feeding. There are, however, two important caveats to consider. First, these studies typically measure fat-free mass, not muscle; as such, broad measurements of fat-free mass may not directly reflect the protein balance of skeletal muscle. Second, the likelihood of substantial muscle loss is almost certainly higher in highly trained individuals attempting to get very lean than in the samples studied to date. Such a population tends to carry more muscle tissue, employ a larger caloric deficit, induce more substantial alterations in anabolic and catabolic hormones, and approach essential body fat levels to a greater extent than the general population, or even a recreationally trained population. For less extreme instances of weight loss, I am of the opinion that time-restricted feeding can be a valuable strategy for people who prefer to eat fewer, larger meals and enjoy the psychological benefit of forgetting about food during long fasting periods. However, early in my bodybuilding career, I implemented this strategy myself during contest preparation. While it fit my schedule and eating preferences quite well at the time, I suspect that a more even meal distribution would have better preserved muscle tissue.
Manipulating the energy deficit within a week or a month
Rather that shifting meal times within a day, some studies have investigated the effects of shifting the days in which calories are consumed. These protocols often encourage people to have anywhere from 1-4 “fasting” days per week in which they either consume a mere 25% of their normal energy intake, or skip out on eating altogether. In the research world, this is known as intermittent fasting or intermittent energy restriction (note the terminology discrepancy: the fitness industry often uses “intermittent fasting” to describe time-restricted feeding, while the research world uses “intermittent fasting” to describe the implementation of one or more weekly fasting days). In comparison to time-restricted feeding, many studies have investigated the weight loss effects of intermittent energy restriction. As displayed in Figure 8, there are a few types of protocols for intermittent energy restriction; the most common include alternate day fasting, fasting for a two-day period, or including two fasts per week on non-consecutive days. Multiple meta-analyses have investigated the efficacy of these interventions in comparison to normal, continuous energy restriction, all with slightly different inclusion criteria and analytical approaches. All three of these meta-analyses have indicated that intermittent energy restriction strategies, using a variety of fasting protocols, do not lead to greater weight loss. However, they also don’t seem to be substantially worse (at least in untrained subjects). While I maintain concerns about lean mass retention when using prolonged fasting periods during fairly extreme dieting, intermittent fasting strategies that implement short (16-20 hours) or long (24+ hours) fasting windows can be effectively used by dieters who prefer the way these protocols fit their daily schedule or satiety preferences.
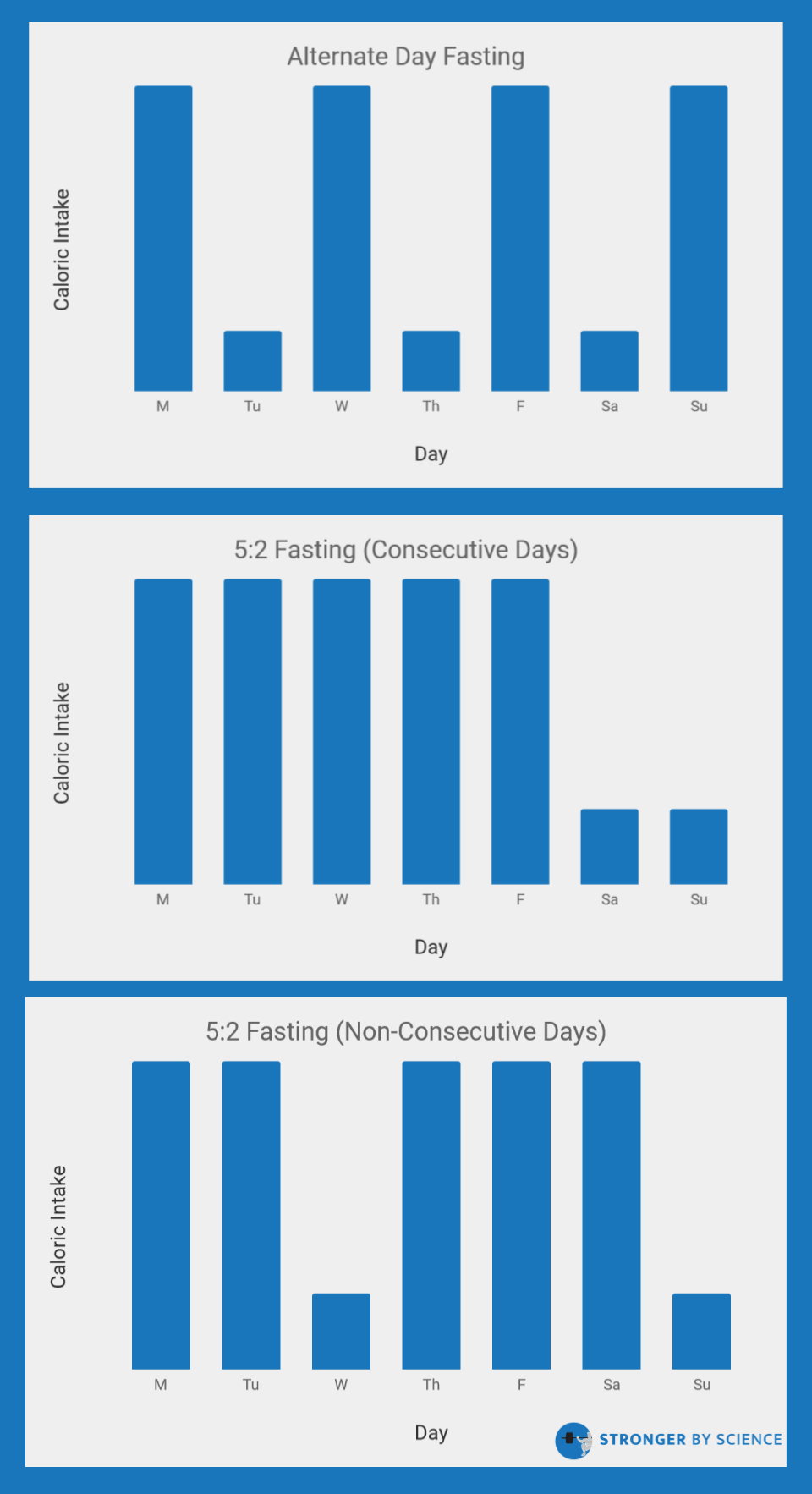
Implementation of “refeeds” offer yet another option for manipulating energy intake throughout the week. A refeed involves acutely increasing caloric intake, usually by specifically increasing carbohydrate intake, and usually for no more than a day or two at a time. Research has shown that this approach is commonly used by competitive physique athletes, either through structured refeeds or unstructured “cheat meals.” The purported benefits of refeeds are multifaceted; by acutely increasing carbohydrate (and energy) intake, one could potentially boost leptin and thyroid hormone levels, acutely increase energy expenditure, enhance muscle and liver glycogen content, and provide a welcome reprieve from the low-carb food menu that often accompanies contest preparation, particularly in the later stages. There is absolutely evidence supporting acute effects of refeeding on hormones and energy expenditure; one study found that a three-day carbohydrate refeed, but not a fat refeed, increased leptin by 28% and total daily energy expenditure by 7%. However, there is a big problem regarding the applicability of this protocol: it requires three days of 40% overfeeding, so that 7% increase in energy expenditure is entirely overshadowed by a huge caloric surplus. Viewed purely as a weight loss strategy, this is taking one step forward and several steps backward. Our study on physique athletes observed a marked increase in resting energy expenditure when comparing values measured shortly before competition to values obtained shortly after, which coincided with an increase in food intake. Again, this marked increase in energy expenditure was nowhere near enough to compensate for the increase in calorie intake required to induce it.
When it comes to more practical approaches to refeeding, Bill Campbell’s lab from the University of South Florida has published a couple of abstracts from a seven-week study that provide some useful data. The study imposed a 25% calorie deficit for a group of resistance-trained individuals; one group had the same caloric intake every day, while the other had two consecutive, high-carbohydrate refeed days. In order to accommodate this two-day increase in energy intake, the deficit imposed on the other five days of the week was increased to ensure that both groups had the same overall energy deficit. Results from the abstracts should be taken with a grain of salt; abstracts are not reviewed as rigorously as full manuscripts, and the primary analysis for each abstract found no group by time interactions. However, some follow-up pairwise comparisons suggested that refeeding may have conferred slight benefits when it comes to maintenance of resting metabolic rate and the retention of fat-free mass.
There is a possibility that longer durations of increased calorie intake may impart more protective effects, and a few studies shed light on this possibility. An eight-week study compared standard continuous energy restriction with an intermittent protocol that had subjects alternate between one week of energy restriction and one week of unrestricted eating. The groups lost somewhat similar amounts of weight over the eight weeks (3.2kg in the continuous group versus 2.0kg in the intermittent group). While the weight loss difference between groups was almost statistically significant (favoring the continuous group), the continuous group spent twice as much time in an energy deficit; as such, the intermittent protocol offered fairly similar rewards, but at a lower overall cost to the dieter enjoying breaks every other week. A separate six-week study compared a standard weight loss diet to a nonlinear approach, in which participants repeated two-week cycles of a 45% calorie reduction for 11 straight days followed by three days of self-selected eating. In the standard diet condition, participants simply reduced their caloric intake by 55% and ate this amount every day. After the six-week weight loss phase, there was a four-week follow-up period in which subjects ate around maintenance calories. In this study, the group with three-day “refeeds” kept their resting energy expenditure a little bit higher than the other group, and lost a little more weight (expressed as a percentage of weight lost), despite averaging higher daily caloric intake throughout the study.
More recently, a study investigated the effects of even longer diet breaks, with the intermittent group alternating between two weeks of dieting and two weeks of eating at maintenance. That’s a really important distinction; the other studies just allowed subjects to self-select food intake during their “break,” whereas this study carefully fed them enough to reach, but not exceed, maintenance calories. Comparatively speaking, this study implemented the highest degree of control. Calorie intakes were consistently updated based on serial measurements of metabolic rate, and meals were cooked and provided by dietitians associated with the study. In order to make a more fair comparison of the diets, the intermittent group dieted for 30 weeks, while the continuous group dieted for 16; this way, the breaks were incorporated, but both groups spent 16 weeks in the same caloric deficit. Results showed that the intermittent group lost significantly more fat (12.3kg versus 8.0kg). Reductions in resting energy expenditure were similar in absolute terms, but the groups clearly had divergent changes in body composition. After adjusting for fat mass and fat-free mass, the continuous group experienced a significantly larger drop in resting energy expenditure than the intermittent group.
Even longer diet breaks have been implemented in the literature. For example, a 2010 abstract implemented a five-month intervention in which subjects consumed 1,200 Calories for a week, 1,500 Calories for three weeks, 2,200 Calories for four weeks, then repeated the cycle. By the end of month five, participants had lost an average of almost 5kg, while avoiding significant reductions in resting energy expenditure. This study did not have a comparator group undergoing continuous weight loss, but it does offer an extreme example of the dilemma we face with the concept of refeeds or diet breaks. Refeeds and diet breaks appear to have the capacity to attenuate metabolic adaptation to some extent, but they also dramatically extend the timeline of the diet. As a result, there is a cost-to-benefit ratio that must be considered when determining the frequency and duration of refeeds or diet breaks.
If you’re keeping your caloric intake within a reasonable range (i.e., not dramatically overeating), a once-weekly refeed probably isn’t going to be enough to make a meaningful effect. If you implement four refeeds per week, you’re mostly not on a diet, from a mathematical perspective, and it’s hard to make your weekly caloric deficit large enough to promote substantial weight loss. To keep fat loss progressing forward and attenuate metabolic adaptation, two refeeds per week is probably the number most people should aim for. Refeeds should absolutely emphasize carbohydrate intake, and caloric intake should be increased to right around maintenance level. Some argue that these refeeds should be placed on consecutive days, to allow a longer duration of time to physiologically “adjust” to being out of a deficit. Others argue that refeeds should be staggered throughout the week; they contend that metabolic adaptations are exacerbated by spending several days in a row in an energy deficit. A large portion of the leptin drop observed with weight loss occurs in the first week. For example, a study demonstrated that a huge fall in leptin occurs during the first four days of energy restriction, followed by a much slower decline over the following 24 days. By providing a refeed every three or four days, you could potentially provide a timely pulse of energy balance to keep metabolic adaptation at bay.
At this point, you can’t really say either approach is definitely wrong. I lean toward consecutive refeeds rather than splitting them up for a few reasons. From a theoretical perspective, we have operationalized metabolic adaptation as a coordinated response to prevent starvation. It’s hard to imagine that a single meal (or a couple meals) would convince our hypothalamus that, despite our prolonged confrontation with starvation, our circumstances have completely changed, such that we would rapidly override one of our most important survival responses. If we draw inferences from some tangentially relevant data, alternate-day fasting can be used to assess the effects of short but frequent refeeds. In rats, alternating between one day of fasting and one day of eating increased leptin, which had favorable effects on weight control and food intake compared to control rats. However, these results are confounded by the fact that one human day equals about 34 rat days, from a biological perspective. In humans, one alternate-day fasting study showed that the intervention did cause weight loss, but reduced leptin by 40%. Another trial compared alternate-day fasting to a “normal” weight loss group; over the eight-week intervention, both groups had similar reductions in weight, leptin, and resting metabolic rate. It’s also important to note that the rise in leptin after consuming a meal is not immediate; according to one study, it’s not until about the fifth hour after a meal that leptin levels appreciably rise, and they continue to rise thereafter. Studies generally show that leptin reductions from short-term energy restriction can be restored within about 12-24 hours of refeeding, but leptin levels also begin falling again within hours of the return to energy restriction. This is coupled with the facts that leptin clearance is especially rapid in lean people, and that many of the downstream benefits we hope to obtain from a leptin spike require multiple-step processes that take some time to play out. With a one-day refeed, I question exactly how much of an opportunity we would have to physiologically capitalize from any observed alteration in leptin. Finally, and most importantly, I’ve actually seen research with two-day refeeds done in lean, resistance-trained people, and it produced modest but favorable results. Once one decides to implement refeeds on consecutive days, the lines between refeeds and diet breaks become blurred. How much time is sufficient? Two days? A week? Two weeks?
There are a lot of contextual factors that determine how refeeds or diet breaks are most effectively used, but it’d be intellectually lazy to leave the answer at, “it depends.” For general weight loss that is intended to be permanent, a two-week diet break could be very doable, and the results from the MATADOR study implementing this technique are quite strong. A bodybuilder that is ahead of schedule, or willing to dedicate to a more prolonged contest prep period, can probably commit to a one-week break implemented every 3-4 weeks, or implemented as needed. If you’re an early-career bodybuilder with plenty of room to grow before you reach your genetic potential for muscularity, you’ll have to carefully consider how much of your year you’d like to spend cutting; while a seasoned pro near their genetic limit ought to take their time, an amateur with plenty of mass to gain should probably avoid excessively long preps. If you’re behind schedule and unable to accommodate weeklong pauses, more frequent two-day refeeds might be the preferred option. If you’re more interested in some of the “other” benefits of refeeding (such as a brief psychological break, flexibility with high-carbohydrate foods, or glycogen replenishment prior to a particularly important training bout), a refeed every 3-4 days could make sense for you. Each type of refeed or diet break is a slightly different tool with slightly different pros and cons. According to a published protocol, we should have some more relevant research at some point in the near future. Until then, we’re left with a few conclusions: refeeds and diet breaks are viable tools to help attenuate metabolic adaptation, the benefit of attenuating metabolic adaptation comes with the cost of extending the total duration of weight loss, and the “ideal” manipulation of deficit days and maintenance days isn’t fully understood at this time.
Balancing Macros To Maintain Muscle and Performance
Dropping Protein
Moving beyond strategies to target the root causes of metabolic adaptation, we have opportunities to alter our dietary intakes to manage some of the secondary effects resulting from metabolic adaptation. Calories must be reduced for the diet to promote weight loss, but there are unique drawbacks associated with lowering intakes of each individual macronutrient.
When evaluating the success of a weight loss diet, the two primary outcomes of interest are: 1) how much weight has been lost, and 2) how much of the weight lost was actually fat mass. In a short-term study (one week) back in 1988, Walberg et al compared isocaloric diets with 0.8 g/kg/day or 1.6 g/kg/day of protein. While the study was almost certainly too short to detect meaningful body composition differences using underwater weighing, the nitrogen balance analysis revealed that the lower protein group was is negative nitrogen balance, with the higher protein group in positive nitrogen balance. These changes in nitrogen balance are consistent with reduced breakdown of lean tissues in the high protein group. More recently, Mettler et al followed up with a slightly longer study, in which 20 resistance-trained athletes were randomly assigned to a high-protein weight loss diet (2.3 g/kg/day of protein) or a control weight loss diet (1.0 g/kg/day of protein) for two weeks. At first glance, the control diet seemed to be favorable, as they lost more weight during the brief weight loss diet. However, a closer look would reveal that fat loss was virtually identical between the two groups, and the difference in weight loss was due to a significantly greater loss of lean mass in the control group. Eric Helms published a review on the role of protein intake during energy restriction in resistance trained (and lean) athletes; in line with the findings of Walberg et al and Mettler et al, the collective body of evidence suggests that protein intake must be prioritized for the retention of lean mass under these circumstances. As noted in a review by Paddon-Jones et al, protein also carries some additional fringe benefits with it. Compared to carbohydrate and fat, protein features a substantially larger thermic effect of feeding and induces greater feelings of satiety on a calorie-for-calorie basis. As Helms points out in his protein review, there is a high likelihood that protein needs are not just important, but actually higher than normal when lean, resistance-trained individuals are engaged in weight loss. While typical protein intake recommendations are around 1.4-2.0 g/kg/day, this fails to account for hypocaloric conditions, and inherently assumes a fairly “typical” body fat level. For these reasons, Helms scales his recommendations to kilograms of fat-free mass and recommends daily protein intakes of 2.3-3.1 g/kg of fat-free mass during energy restriction.
Dropping Fat
Changes in sex hormone levels may be a contributing factor to the loss of lean mass during weight loss. Beyond weight loss, these sex hormones certainly contribute to the unpleasant reproductive side effects of extreme weight loss, and it would be fantastic to attenuate sex hormone reductions if possible. Over the years, several studies have identified a link between dietary fat intake and sex hormone levels. For example, reducing dietary fat intake from 40% of calories to 25% for six weeks led to significant reductions in total testosterone and free testosterone in healthy men. Another study using male subjects instructed them to consume a diet including 100 g/day of fat for two weeks, followed by a drop to less than 20 g/day for two more weeks. This drastic drop in fat intake resulted in a combination of increased sex hormone-binding globulin and reduced free testosterone, and both changes are associated with a reduction in the biological activity of testosterone. In male strength athletes, dietary fat intake has also been correlated with both resting testosterone levels and the post-exercise testosterone response to heavy resistance exercise. Further, the link between dietary fat and sex hormones is not restricted to men. An intensive two-year trial evaluated the effects of reducing dietary fat to 15% of calories in premenopausal women; results showed that women assigned to the low-fat diet ended the trial with significantly lower levels of estradiol and progesterone and 7% higher (but not significantly different) levels of follicle-stimulating hormone, which plays a role in the regulation of estradiol production and the menstrual cycle. Just as low-fat diets appear to have a suppressive effect on male testosterone, this finding would suggest that analogous changes occur in women, with suppressed female sex hormone levels that could potentially interfere with menstrual cycle regularity and reproductive function.
Unfortunately, dietary fat intake is only one of the factors influencing sex hormone levels, and it’s almost certainly not the biggest factor. Long-term caloric restriction itself has been associated with lower sex hormone levels in males, in a manner that did not appear to be attributable to age, body fat percentage, or the proportion of calories from dietary fat. Research in anorexic females has shown that, even among anorexic patients who generally have low BMI and chronically restrict energy intake, body fat percentage seems to be an important independent predictor of developing menstrual cycle dysfunction and of eventually recovering from menstrual cycle dysfunction after it has occurred. This is coupled with the observations that low testosterone is very consistently observed in males with anorexia and in published male bodybuilding case studies, despite the wide range of potential dietary tactics that could be employed to reach such low levels of body fat. In addition, testosterone recovery in bodybuilding case studies often takes several months, with recovery coinciding more closely with the restoration of body fat levels than increases in dietary fat intake. Taken together, the available evidence suggests that sex hormones are reduced to some extent by caloric restriction and extreme leanness, which are inherently unavoidable when the goal is to get really lean. So, while it is prudent to avoid exacerbating the downregulation of sex hormones by excessive reduction of dietary fat, your chances of manipulating fat intake to completely abolish drops in sex hormones are slim.
Dropping Carbohydrate
There are also some downsides to reducing carbohydrate intake. A few published observations in bodybuilders lend some evidence for the role of carbohydrates in supporting bodybuilding success. An observational study in natural bodybuilders previously noted that more successful competitors tended to report higher carbohydrate intakes at the beginning of preparation compared to less successful competitors. The primary limitation here, aside from the observational study design, is that carbohydrate intakes were only significantly different at the start of the diet, but not the end. Theoretically, if the plan was to identify nutrition strategies that facilitate a successful contest prep, the dietary differences toward the middle and/or end of the diet would be more informative than those at the start. Maestu et al observed that individuals who had the largest insulin reductions throughout contest prep also tended to lose the most lean mass. Given the robust insulin release observed in response to carbohydrate intake, one might infer that a higher carbohydrate intake would help preserve lean tissue during a weight loss diet. However, it is important to note that dietary protein can also be quite insulinemic, and insulin levels also correlated with the loss of fat mass in this study. So, it is possible that the people who lost less fat mass had higher insulin levels and retained more lean mass because they simply didn’t get as lean as the other competitors in the sample.
Looking beyond these cloudy instances of indirect evidence, there are more controlled studies that show some direct disadvantages of slashing carbs from the diet. Whether we are resting or sprinting, we rely on a combination of three energy systems to supply the ATP required. At rest, the rate of ATP demand is pretty low, so the aerobic energy system is able to provide ample ATP, primarily by breaking down fat. As we begin to engage in activities of higher relative intensity, the contributions of the anaerobic (phosphagen and glycolytic) energy systems increase. This is important because the glycolytic energy system makes a very substantial contribution to fueling resistance exercise, and by definition this energy system relies on carbohydrate as the substrate for ATP synthesis. As such, it should be no surprise that studies have shown carbohydrate restriction to impair resistance exercise performance. One study put participants through a glycogen-depleting workout, had them restrict carbohydrate (1.2 g/kg/day) intake for 48 hours, then assessed resistance exercise performance. For the three-set squat protocol at 80% of the one-rep maximum load, carbohydrate restriction caused participants to perform significantly fewer repetitions during sets one and two. In addition, the previous study by Walberg et al showed that high protein diets maintained positive protein balance, but protein was kept high by reducing carbohydrate intake. An unintended consequence was that, despite favorable effects on protein balance, the high protein/low carbohydrate group had a reduction in quadriceps muscle endurance compared to the group with lower protein and higher carbohydrate intake. These applied performance outcomes are supported by well-controlled in vitro studies investigating the link between carbohydrate and muscle force production. The sarcoplasmic reticulum is a calcium-storing structure found within muscle cells, and the release of calcium from the sarcoplasmic reticulum is a critical step mediating a muscle fiber’s ability to produce force. In instances when carbohydrate intake is insufficient to replace muscle glycogen use, the glycogen stores adjacent to the sarcoplasmic reticulum become depleted. Several studies have shown that this results in lower calcium release from the sarcoplasmic reticulum, leading to more rapid muscle fatigue and earlier reductions in force production. As such, strategically utilizing carbohydrate intake to attenuate the magnitude of muscle glycogen depletion during energy restriction can be viewed as a preferred dietary strategy to preserve performance in the gym.
Striking a Balance
At this point, we’ve reviewed the evidence suggesting that your weight loss diet should be high in protein, high in fat, and high in carbohydrates in order to maintain muscle, attenuate metabolic adaptation and related side effects, and support performance in the gym. Good luck trying to make that work while successfully losing weight.
Once we’ve acknowledged that perfection is out the window, the discussion shifts to making this puzzle work as best we can. Sufficient protein intake is simply non-negotiable, and recommendations suggest this should be set around 2.3-3.1 g/kg of fat-free mass, which is often up around 2 g/kg of total body mass or higher (depending on how lean you are). Once protein is set, it’s important to set a “basement value” for fat, or a level of fat intake you simply are not willing to go below. This is a bit of a balancing act; we need to remove calories from somewhere, and fat calories are a viable option for removal. However, going too low with fat will likely exacerbate reductions in sex hormones during weight loss, and we need enough fat to obtain sufficient essential fatty acids, promote the absorption of fat-soluble vitamins, and keep the diet reasonably palatable. For most individuals, fat intakes really shouldn’t drop below around 0.6-0.7 g/kg of total body mass per day. Once these minimums are set for protein and fat, the rest of your calories are going to come from some mixture of carbohydrate and fat; in the interest of performance, I prefer for the majority to come from carbohydrate. These macronutrient boundaries have little chance of completely attenuating adaptive thermogenesis, hormone fluctuations, performance decrements, or the loss of lean mass. They’re just the best we can do with what evolution gave us.
Managing Cardio For Successful Weight Loss
Weight loss is dictated by creating an energy deficit, and energy intake is only half of the equation. Weight loss diets are typically paired with increases in cardiovascular exercise, and the inclusion of cardio is almost always part of the approach for physique athletes. Many studies have been conducted to investigate the effects of adding cardio to a resistance training program. In some instances, the additional cardio blunts adaptations to resistance exercise, which is known as the interference effect (i.e., the cardio interferes with adaptations to the resistance training program). In short, the root of this interference is attributed to the stimulation of conflicting cell signaling events that underpin training adaptations to strength and endurance training, and additional fatigue induced by additive endurance exercise. Under normal circumstances, the interference effect impacts power outcomes more than it impacts strength, and impacts strength more than it impacts hypertrophy. However, a number of contextual factors dictate the likelihood of observing a substantial interference effect. Most notably, as one might expect, more cardio seems to equate to more interference. Studies implementing two or fewer sessions of cardio per week have generally failed to observe substantial interference, whereas studies implementing three or more sessions of cardio per week often observe statistically significant impairment of resistance training adaptations. However, there is currently insufficient evidence to determine if it is the frequency or volume of cardio that is driving these outcomes, as high-frequency cardio programs also tend to be higher in volume in the studies published to date.
For physique athletes, the combined load of resistance training and cardio is at the high end of this frequency/volume discussion toward the end of contest prep. In case studies we have documented, it’s fairly typical for both male and female competitors to add up to five or six cardio sessions per week, on top of an independently demanding 5-6 day per week resistance training program. In addition, these athletes are fighting an uphill battle in terms of hormones. As discussed in Part 1, weight loss attempts in lean individuals are often accompanied by decreases in testosterone and increases in cortisol. For years, the testosterone to cortisol (T:C) ratio has been used as an imperfect, but informative, marker of overtraining and impaired recovery. Even in normal-weight athletes with unrestricted caloric intake, excessive training loads often drive the T:C ratio downward to a level that is associated with an unfavorable “anabolic-catabolic balance.” The recovery of physique athletes is also threatened by insufficient energy intake to maintain body weight (which is unavoidable) and commonly observed issues obtaining quality sleep (which is pretty hard to fix). Taken together, the circumstances surrounding weight loss, particularly in more extreme cases like physique athlete contest preparation, present conditions in which the likelihood of an interference effect is increased. Moreover, careless implementation of excessive cardio has great potential to exacerbate some of the unfavorable effects observed with metabolic adaptation, especially those linked to fatigue and hormone fluctuations.
To avoid exacerbating some of these issues, cardio should be thoughtfully and carefully implemented as part of the weight loss plan. Unfortunately we have to make some very broad observations and some tenuous conclusions when viewing the concurrent training literature, because there is an immense variety of factors that can fluctuate within a single study design. For example, I might want to make an inference about how the intensity of cardio affects strength in the next resistance training bout. A study might shed some insight on this question, but there will inevitably be confounding factors to consider (such as the modality of cardio, duration of the overall bouts, frequency, overall training loads of both resistance training and cardio components within the study, training status of subjects, sex of subjects, duration of the study, and the time elapsed between cardio and strength testing, just to name a few). Whenever you change one factor in this type of study, there tends to be a ripple effect that requires alteration to some other factor(s). Nonetheless, a review by Fyfe et al helps to consolidate some of the information currently available from concurrent training studies, and some general recommendations can be made for people who wish to be cautious with their cardio implementation. Certainly the overall load of training must be managed, and dieters should err on the side of doing as little cardio as they can get away with while still supporting fat loss (and general cardiovascular health, if that’s important to you). There are also temporal considerations; following endurance exercise, force production of the trained musculature is typically impaired for at least six hours. The evidence would suggest that the most prudent approach would be to perform cardio and resistance training on separate days, if possible. When cardio and resistance training must be performed in the same session, it would be preferable to prioritize resistance training first, which seems to be as effective or slightly more effective for attenuating interference than performing resistance training after cardio (we’re talking about the actual bout of cardio here, not a quick warm-up to get moving before lifting). When it comes to intensity, things are a bit complicated; high-intensity bouts cut down on the overall duration of cardio, but really intense bouts call for a level of physiological arousal that can be quite exhausting and may induce more residual neuromuscular fatigue. Looking at the literature, it also appears that running is associated with more interference than cycling, which may relate to less muscle damage, less residual neuromuscular fatigue, or less robust stimulation of interfering molecular pathways with cycling exercise.
Finally, it’d be shortsighted to make cardio-related decisions without first considering the preferences of the dieter. While a physique athlete should never aim to exclusively run their way to contest shape, physique athletes can succeed within a wide range of cardio implementation strategies, and the restrictions on non-physique athletes are even less restrictive. Some people hate cardio, and that shouldn’t be ignored. Dieting for weight loss is already unpleasant enough, even when done well; the cumulative psychological burden of spending several hours per week doing something you dread should not be overlooked, as weight loss success is contingent on both psychology and physiology. While I’m personally pretty neutral about cardio in terms of enjoyment, it tends to markedly increase my hunger. While this doesn’t happen to everybody, I’m also not alone; research suggests that there is a great deal of variability with respect to hunger and other compensatory responses to cardio. Conversely, some people quite enjoy cardio (I think — I’ve never met one, but I’ve been assured they exist). If that is the case, and they aren’t exacerbating their hunger or aggravating injuries in the process, then reasonable amounts of cardio should absolutely be used as part of the plan to promote an energy deficit.
Other factors to consider
When it comes to attenuating the effects of metabolic adaptation, the most influential factors under our control have been covered in previous sections (rate of weight loss, refeeds and diet breaks, macronutrient intakes, and management of cardio). There are a few other factors that are a little harder to manage or that make a slightly smaller impact but deserve acknowledgement nonetheless.
Sleep and stress
Sleep is important for anyone with strength- or physique-related ambitions. Aside from the negative effects of sleep deprivation on cognition, mood state, and general physical performance, there is evidence to suggest that three days of partial sleep restriction impairs bench press, leg press, and deadlift strength. While there is a surprising lack of sleep research in the area of resistance training recovery, it’s a safe assumption that sleep is an important component. As a fairly obvious, surface-level observation, lack of sleep seems to impair readiness to train and interfere with voluntary effort. Sleep restriction also has metabolic and hormonal effects that can exacerbate aspects of metabolic adaptation. Insufficient sleep impairs glucose tolerance and insulin sensitivity, while increasing cortisol and ghrelin levels, decreasing leptin, and increasing hunger and appetite. Sleep restriction also shifts food preferences toward more calorie-dense, hedonically rewarding options, thereby threatening dietary adherence. Sleep-induced increases in cortisol have been observed in conjunction with reductions in testosterone and IGF-1, prompting researchers to speculate that insufficient sleep may be linked with muscle loss and impaired muscle recovery. In support of these mechanistic findings, a small crossover study found that individuals lost less fat mass and more fat-free mass during caloric restriction when they slept 5.5 hours per night compared to 8.5 hours per night.
Unfortunately, sleep is rarely discussed in the bodybuilding literature. One of our case studies evaluated sleep, both using objective and subjective assessments. While the accelerometer-based objective measurement showed no meaningful change, subjective assessments showed that sleep quality suffered toward the end of contest preparation. It’s possible that the accelerometer simply wasn’t equipped to accommodate the fact that bodybuilders in contest prep constrain their non-exercise movement to a statue-level degree of stillness. The subjective reduction in perceived sleep quality supports widely reported anecdotes of sleep issues during the later phases of contest prep; even as sleep becomes harder to obtain in more extreme weight loss attempts, an earnest effort should be made to obtain it. Strategies to help promote sleep quality include limiting blue light exposure within 1-3 hours of intended sleep onset and limiting the intake of stimulants with long half-lives (such as caffeine) late in the afternoon and evening. Research also suggests that diets relatively high in protein and carbohydrate, with low or moderate fat intake, support high quality sleep, as does consumption of a carbohydrate-rich meal in the evening and avoidance of going to sleep while hungry. Unfortunately, these are luxuries that might not be feasible on a more intense weight loss diet. More practically speaking, a handful of supplements (including melatonin, tryptophan, valerian, and magnesium) are purported to have beneficial effects on sleep.
It’s also prudent to discuss the effect of stress in the context of metabolic adaptation, as stress, sleep, and metabolism are all inter-related. Stress can induce sleep loss, sleep deprivation can increase subjective stress and anxiety, and both subjective stress and sleep deprivation can influence metabolism. The effects of stress are primarily dictated by cortisol, with downstream effects on testosterone, leptin, ghrelin, appetite, and a shift toward more hedonic and calorically dense food preferences. Excessive stress yields outcomes quite similar to sleep deprivation, and the two often go hand-in-hand. Unfortunately, stress can sometimes be hard to avoid during a fairly intense weight loss phase (or life in general), especially if the stress of an impending competition is involved. Aside from general stress management techniques, the most direct approaches to mitigate excessive stress during weight loss include prioritizing effective sleep habits, allowing a flexible timeline for your weight loss goal, and avoiding diet and exercise strategies that cause stress because you hate doing them so much. Adaptogen supplements, such as rhodiola rosea and ashwagandha, may also play a role in attenuating the effects of high stress levels.
Supplements
When it comes to practical strategies for attenuating metabolic adaptation, I would be remiss to neglect the potential applications of supplements altogether. However, I broach the subject with great trepidation, as I’ll explain shortly.
There are indeed some supplements and/or over-the-counter drugs that promote energy expenditure and fat loss, such as ephedrine, p-synephrine (bitter orange), yohimbine, caffeine, nicotine, and capsaicin. Each one comes with drawbacks. Ephedrine is banned in many countries, including the United States, due to the receipt of several severe adverse event notifications. P-synephrine is essentially a less potent version of ephedrine; its side effects are less potent, but so are its effects on energy expenditure. Until more research on p-synephrine becomes available, I’m not entirely convinced that its effects are meaningful enough to support its use. Yohimbine is known to cause anxiety symptoms in many people, interacts with several drugs, has some quality control issues with regard to dosing in products, and ought to be used in conjunction with fasted cardio (which I rarely recommend) to maximize its benefit. Caffeine’s effect on metabolic rate is fairly modest, and it’s quite easy to go “overboard” with caffeine intake as you start becoming habituated to its effects. It’s not uncommon to hear about dieting individuals that are taking in pretty hefty caffeine doses daily (sometimes over 1,000mg per day), and unsurprisingly experiencing restlessness, anxiety, headaches, or insomnia as a result. Nicotine is quite addictive, and it seems a bit unethical to 1) advocate the off-label use of a drug for unintended purposes, such as a weight loss aid, and 2) advocate the use of an addictive substance that, despite having a far more favorable safety profile than tobacco, may still have independent adverse health effects of its own. Finally, there is capsaicin, the extracted component that gives cayenne and chili powder their “kick,” and capsiate, a less pungent analog of capsaicin. Research does suggest that these chili pepper components increase metabolic rate, especially at higher doses (135-150mg capsaicin or 6-9mg dihydrocapsiate), in a reasonably safe manner. However, the magnitude of this effect is fairly modest, and by no means a miracle strategy for keeping metabolic adaptation at bay. There is also emerging evidence that ephedrine and several dietary components (including capsaicin, resveratrol, curcumin, green tea, berberine, fish oil, conjugated linoleic acid, and all-trans retinoic acid) may activate brown adipose tissue and/or promote the “browning” of adipose tissue, a process by which white fat cells begin behaving more like brown fat cells by increasing uncoupled thermogenesis. However, the evidence for such an effect is quite preliminary and largely derived from non-human research models. So, aside from a morning or pre-workout caffeine boost and generous servings of cayenne powder on some meals, I do not advocate (or implement) any supplement strategies specifically for the purpose of maintaining energy expenditure during weight loss.
Many of the supplements suggested to promote energy expenditure are also purported to attenuate hunger. In addition, some non-thermogenic supplements are thought to suppress appetite or promote satiety, such as ginger, 5-HTP, caralluma fimbriata, and alpha-lipoic acid, among others. For each, high-quality evidence in human subjects is fairly limited. Aside from the use of supplements, there are some very practical dietary strategies to promote satiety and mitigate hunger. Examples include selecting foods with relatively high satiety index values, eating high-volume foods with low caloric density, drinking green tea with meals, and consuming conventional foods with generous amounts of red pepper. While there are some supplements and strategies that may have minor effects on appetite, I generally advise against the premise of aggressively “hiding from hunger” during more extreme weight loss attempts. With small, sustainable weight loss attempts, you may never actually reach a point of substantial hunger. If preparing for a physique competition, hunger will be a reality, and it’s probably best (from a psychological viewpoint) to just embrace that. When you try to plan your whole day around avoiding the threat of hunger, it gives hunger way too much power over your subjective well-being. Hunger is, after all, a natural and appropriate response to the restrictions you’re imposing, so it is remarkably difficult to override and there is no reason to fret over it.
Conclusion
Metabolic adaptation is going to happen, but there are some very practical strategies to help attenuate its effects. Daily protein should generally be set at no less than 2 g/kg of total body mass, and up as high as 2.3-3.1 g/kg of fat-free mass, when in a sizable caloric deficit. Fat intake typically shouldn’t go below 0.6-0.7g/kg of total body mass as a bare minimum value, and dieters should make an effort to prioritize carbohydrate with whatever calories they have left over. Slower rates of weight loss should be preferred; especially for fairly lean individuals, the rate of weight loss should rarely exceed 1% of body weight per week. In the interest of lean mass retention, recovery, and maintenance of sanity, reasonable volumes and frequencies of cardio training should be used to promote an energy deficit. Refeeds and diet breaks may provide additional protections against the effects of metabolic adaptation. Refeeds should be implemented 2-3 times per week, and you can make a reasonably strong (but not indisputable) argument that these should be situated consecutively. When the diet timeline allows, these refeeds could be taken a step further with the implementation of periodic diet breaks, lasting one to two weeks in duration.
That’s a perfectly suitable plan if you aren’t banking on surviving past the end of your weight loss phase. For whatever reason, that’s about as far as most people plan. Fortunately, for the overwhelming majority of dieters, life goes on after you reach your weight loss goal. Much like puberty, it’s a chaotic time; your body is changing, hormones are fluctuating wildly, and it can be a bit stressful if you haven’t been given a warning about what to expect. Part 3 is that warning and offers some strategies to plan for a less chaotic transition to life after weight loss.
Part 3: Life After Weight Loss
For short-term dieting, just about everything works. For long-term dieting, almost nothing works. There are 8-12 week studies showing successful weight loss with just about every approach in the book, but studies suggest that only about 10-20% of individuals are able to maintain substantial weight loss in the long term. If you are dieting to a sustainable body weight and you intend to maintain a large percentage of your weight loss, research generally suggests that maintaining high levels of physical activity and frequent monitoring of body weight are important strategies to promote weight loss maintenance. Even with these strategies, weight loss maintenance proves to be a very challenging endeavor. If you’re only planning to hold your weight loss for a short amount of time (such as dieting for a weight-based athletic event, a photo shoot, or a physique competition), then weight regain is expected. However, since we know weight regain is coming, it should be done intentionally, in a well-planned and thoughtful manner. Understanding the weight regain literature facilitates the formulation of such a plan.
Underwhelming success rates with long-term weight loss have prompted researchers to consider the possibility that metabolic adaptation may persist beyond active weight loss, and into the weight maintenance (or weight regain) period. One small but tightly controlled study sought to determine if energy expenditure remained suppressed during weight loss maintenance. In order to assess this research question, they measured total, resting, and non-resting energy expenditure in three types of people: 1) people at their normal body weight, 2) people who had maintained a 10% body weight reduction for the previous 5-8 weeks, and 3) people who had maintained a 10% body weight reduction for the previous 1-6 years. Even after adjusting for relevant confounding variables, both weight loss groups appeared to have total, resting, and non-resting energy expenditure values that were lower than predicted. When compared to the normal weight group, both weight loss groups had significantly lower total and non-resting energy expenditure values, with no significant difference between recent and sustained weight loss groups.
Another study assessed participants from the Biggest Loser competition six years after achieving dramatic weight loss. Participants had originally lost an average of 58.3kg, and had gained back 41.0kg by the six-year follow-up study. Resting metabolic rate was 704 Calories below baseline, which is 499 Calories lower than predicted. In this study, the degree of resting metabolic rate suppression was correlated with the degree of weight loss that had been maintained, meaning that individuals who maintained more weight loss displayed a greater degree of adaptive thermogenesis. The propensity for weight regain after weight loss is not a coincidental trend; as reviewed by MacLean et al, many of the aspects of metabolic adaptation that oppose active weight loss also predispose us to weight regain, especially if we lost a ton of weight or got really, really lean. As these two studies have shown, some aspects of metabolic adaptation can even persist for years after active weight loss. In the time period immediately following weight loss, we have a body with less metabolically active tissue, emptier adipocytes, more energetically efficient tissues, less leptin, lower levels of thermogenic, anorexigenic, and anabolic hormones, and higher levels of orexigenic and catabolic hormones. It is a body primed for ravenous eating and precipitous fat gain.
Body Fat Overshooting
This predisposition to rapid fat gain can be a bit problematic if we allow it to go completely unchecked. Our adipocytes (fat cells) get smaller when we lose weight, with a negligible change in the number of total adipocytes. As previously discussed, a huge ramification of reduced adipocyte size is that the adipocyte produces less leptin, which drives metabolic adaptation forward. Even beyond leptin, a thorough review by MacLean et al describes numerous ways in which the physiology of adipocytes changes in response to weight loss. Adipocyte gene expression changes, shifting toward a gene expression profile that downregulates energy expenditure, increases appetite, and promotes the storage of nutrients. Smaller (i.e., emptier) adipocytes react to insulin differently; they take up more glucose in response to insulin stimulation, and are less sensitive to insulin-induced suppression of lipid breakdown. They also tend to have lower levels of adipocyte triglyceride lipase (ATGL), hormone sensitive lipase (HSL), and lipoprotein lipase (LPL), which are critical for lipid breakdown. Changes in extracellular matrix structure, sympathetic tone, and circulating thyroid hormone may be underlying many of the changes in the metabolic characteristics of adipocytes, ultimately yielding a physiological environment in which fat storage is promoted.
While weight loss doesn’t alter the number of adipocytes, rapid weight regain might. Ideally, any weight regain would just refill the existing adipocytes, but the altered metabolic environment following weight loss may promote the addition of new fat cells, known as adipocyte hyperplasia (Figure 9). This effect has been observed in overfed rats; while it still requires additional research to demonstrate its relevance to humans, it could be but one of many factors contributing to fat regain after weight loss. As noted in a review by Peos et al, this would be particularly disadvantageous for athletes that complete several weight loss cycles across a career. Addition of new adipocytes increases the overall capacity for fat storage, and allows fat to be stored while the original adipocytes have yet to be fully replenished with energy. In the short-term, this could increase the likelihood that individuals not only regain the fat they lost, but actually gain more fat than they lost. In the long-term, this could yield even more adipocytes with the potential to convey their relative “emptiness” during the next weight loss attempt, potentially amplifying some of the physiological signals that promote metabolic adaptation and oppose weight loss.
Mechanistic rodent data are fascinating, but only if they pan out in humans. While we don’t have much direct evidence of adipocyte hyperplasia in re-fed humans, we do have some intriguing observations of loosely related outcomes. An interesting observation in studies evaluating weight regain is that the early regain phase is characterized by selective storage of fat, with minimal restoration of fat-free mass. This effect, known as post-starvation obesity, has been observed in several previous studies, including the old Biosphere study from Part 1 of this article. This could be related to adipocyte hyperplasia to some degree, but the more prominent driver appears to be the loss of fat-free mass while dieting (Figure 10).
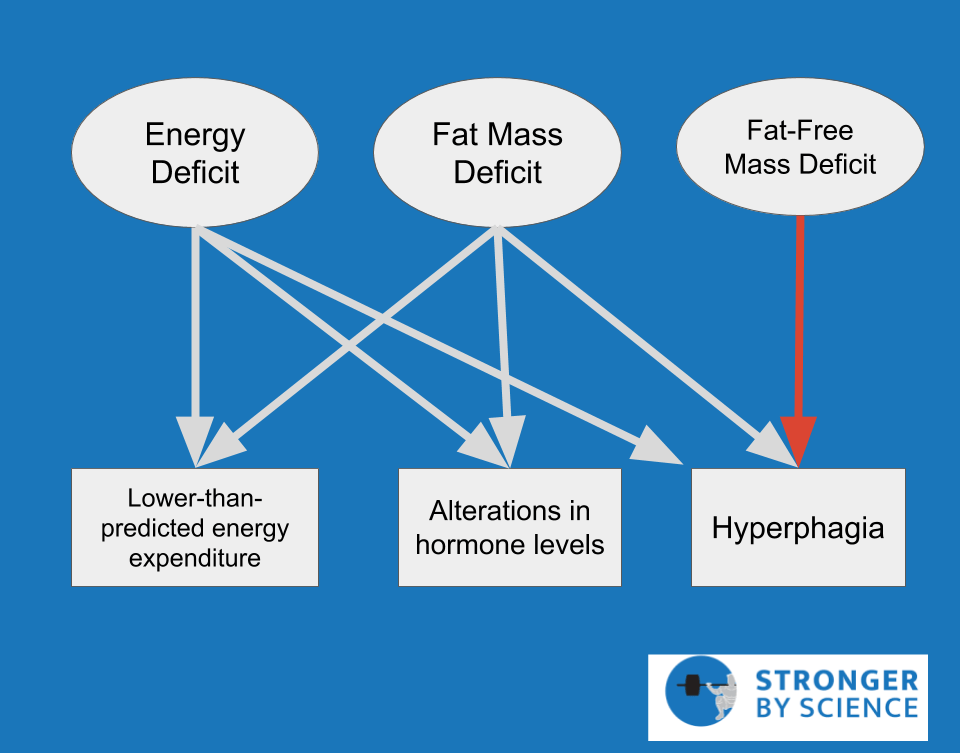
As explained in a paper by Dulloo et al, adaptive suppression of metabolic rate is most closely linked to the existence of an energy deficit and the loss of fat mass; in contrast, hyperphagia is linked most closely to reductions in fat mass and fat-free mass. With weight regain, the energy deficit is eliminated immediately, and fat mass is restored fairly promptly. However, the restoration of fat-free mass takes longer, and it is common for fat mass to be entirely restored to pre-diet levels before fat-free mass is fully restored (for a visualization of this effect using arbitrary numbers, see Figure 11). In this scenario, hyperphagia continues in order to complete the restoration of fat-free mass, and the dieter actually regains more fat than they initially lost. This is known as fat overshooting, and may be driving the results of observational studies reporting that athletes who engaged in repeated cycles of weight loss and weight regain tend to have higher BMIs later in life.
Whether or not adipocyte hyperplasia is an important contributing mechanism in humans, the available human research indicates that the physiological environment following weight loss favors weight gain, fat is preferentially regained in the initial phase of weight regain, restoration of fat-free mass is a comparatively slower process, and it is not uncommon to regain more fat than was initially lost. For physique athletes (or strength/power athletes dieting to fairly low body weights), this has some important implications for managing the post-diet period. First, it suggests that the old-school idea of being “primed for muscle growth,” or banking on some rebound effect of rapid muscle gain after a weight loss diet, is mythical and unsubstantiated. We ran a study on the topic, and results showed quite clearly that the initial, immediate post-show weight gain is largely attributable to increased water weight, and the additional weight gained in the first 4-6 weeks was almost entirely fat mass.
Taken together, all of this evidence presents a dilemma for planning the post-diet phase for physique athletes (or other dieters completing fairly intense weight loss phases). Certainly, reversing the unfavorable effects of metabolic adaptation is high on the priority list, which both improves quality of life and sets the stage for progress toward continued strength and/or hypertrophy goals. That process is going to require some additional calories. However, it’s safe to assume that someone who just completed an arduous fat loss phase would prefer to avoid immediately gaining all of it (or more) back. This is difficult, because we are physiologically primed for fat gain after this type of weight loss, and the added calories in the initial post-diet period appear to be preferentially diverted toward fat cells for storage. In order to promote recovery while attenuating excessive fat gain, two primary approaches for controlled refeeding have emerged: reverse dieting and recovery dieting.
Reverse Dieting Versus Recovery Dieting
I think my co-authors and I were the first researchers to actually acknowledge the term “reverse dieting” in a peer-reviewed paper, but we certainly didn’t coin the term. So, let me begin by giving credit where credit is due. I first heard the term “reverse dieting” attributed to Layne Norton; he definitely popularized the term, but neither he nor I know exactly where it was first used. Based on some digging, it appears that the term “recovery dieting” is attributable to Jeff Alberts and the 3D Muscle Journey crew. These could be misattributions, and I apologize if they are, but I tried.
You can’t really search the research literature for an agreed-upon definition for these terms, so I’m going to present some operational definitions that reflect my personal conceptualization of each term (note: these are my interpretations, so I am not putting words in the mouth of the individuals who coined these terms). The general premise with reverse dieting is that calories, mainly from carbohydrate and fat, are incrementally added back to the diet after the weight loss goal has been achieved. As the calories slowly increase, the dieter transitions from a deficit to maintenance to a surplus in a controlled and methodical manner. As calories are increased in a gradual, stepwise fashion, metabolic adaptations are reversed over the course of this process. As a result, energy expenditure increases as caloric intake is increased, which prevents rapid fat accumulation out of the gate. Theoretically, this would allow the dieter to begin their offseason with substantially higher caloric intake than during their weight loss phase, but with a fairly low level of body fat. Recovery dieting is quite similar, with somewhat minor differences in the implementation. It seems as if recovery dieting was essentially introduced in response to excessively slow implementations of reverse dieting; as a result, recovery diets often include a very deliberate jump to a small caloric surplus immediately after the weight loss phase ends, and the approach generally allows a quicker rise in caloric intake and more directly embraces reasonable levels (and rates) of fat regain early in the process.
I don’t see much value in pitting these approaches against each other to determine which is best. Frankly, I struggle to see “reverse diet versus recovery diet” as anything but a false dichotomy. Both approaches seek to address the dilemma of promoting recovery while mitigating excessive fat storage and weight regain. If we were to construct a Venn diagram, these terms would have a lot more overlap than exclusivity. There might be subtle differences with regard to how quickly calories are added, but there are plenty of factors to consider when determining how much weight should be regained, and how quickly. I will certainly acknowledge that a really slow reverse diet could theoretically leave the dieter in a slight caloric deficit for weeks after the intended end of the weight loss diet; this would serve only to delay even the most basic and immediate aspects of recovery, and make their life unnecessarily difficult. Furthermore, an attempt to stay too lean during the offseason would likely inhibit an individual’s ability to fully restore normal hormone levels, reverse the hyperphagia induced from dieting, and make strength and hypertrophy gains in the offseason. It’s also relevant to note that a very slow, very strict reverse diet is unequivocally more challenging and psychologically draining than the most brutal of contest preps. How do you reasonably convince yourself to exercise that level of dietary restraint when it defies all of your physiological cues, and you don’t intend to step on stage for another 24 months?
These theoretical shortcomings exist, so the ideas reinforced by the emergence of recovery dieting are helpful and valid. Some might say that, by acknowledging the potential shortcomings of an extremely slow reverse diet, I have a preference for recovery dieting by default. However, I don’t believe that the concept of reverse dieting necessarily requires such an extreme approach, and I’m not sure how many people are actually willing and able to implement this type of protocol after achieving stage-ready levels of body fat. Frankly, I think both approaches serve to reinforce some very important principles for how to approach the diet after weight loss has occurred. So, rather than construct extreme examples of each approach and have them battle it out, I would much prefer to outline some principles for the post-diet period.
If your diet is over, get the hell out of a deficit, immediately. Beyond that point, any time spent in a deficit is wasted recovery time. If that puts me in the “anti-reverse-dieting” camp, then so be it. Based on this reasoning, I don’t really see a lot of utility in using nonlinear approaches like refeeds or diet breaks; every day should be a surplus, to some degree. If you want some extra special HUGE surplus days to accommodate your eating preferences, and that’s why you’re using a nonlinear approach to calorie distribution, go for it, as long as the “low calorie” days keep you at or above maintenance calories. If you are planning to increase calories slowly enough to create a super-charged metabolic rate, don’t bother. The premise of fabricating a new metabolic rate from scratch is implausible. We only have the capacity to restore a transiently suppressed level of energy expenditure, and we have two primary tools to use: more calories, and more time spent in a surplus. There are certainly instances in which people slowly raise calories and manage to reach fairly high caloric intakes while staying lean, but there are explanations for this that do not require wizardry as an assumption. In the initial stages of slow overfeeding, they are simply fixing an impairment. Their metabolic rate is lower than it should be at the start, and they restore it to its normal level (and, for some people, “normal” is higher than others). After the impairment of energy expenditure is restored, there is also the potential for the opposite effect to occur; in response to overfeeding, some people dramatically increase their non-exercise activity thermogenesis to prevent weight gain. There is a great deal of variability between people for this response; as you might imagine, individuals prone to weight gain typically have a very minor increase in NEAT when they are overfed, while individuals resistant to weight gain display large increases. Furthermore, the individuals who are able to slowly work toward high caloric intakes while maintaining almost-stage-ready body composition are still fairly miserable and unrecovered, for the most part. From a very basic viewpoint, recovery can be viewed in three stages: elimination of the calorie deficit, restoration of reasonably sufficient fat mass levels, and restoration of lean mass. Someone who clings to competition-level body fat is unlikely to achieve fully comprehensive recovery from the multifaceted barrage of metabolic adaptation, and unlikely to reach a high enough body fat level (or energy surplus) to support substantial hypertrophy and strength improvements in the offseason.
Conversely, if you’re planning to rapidly overfeed to capitalize on a quick rebound of muscle gain, don’t bother. As previously discussed, the body is effectively primed for fat accretion, but lean mass gains are hard to come by in this scenario. To optimize the ratio of weight that is regained as lean mass (within a realistic range), the best bet is to get the body into a caloric surplus, nudge the body toward recovery with incremental caloric increases that promote positive energy balance and fat mass restoration at a conservative pace, while engaging in a challenging and progressive resistance training program. Rapid fat gain would probably expedite the recovery process, but would come at the cost of an elevated ratio of fat gain to lean tissue gain and an increased likelihood of fat overshooting.
So, the deficit should be eliminated immediately, and caloric intake should be gradually increased. Exactly how quickly calories should be increased depends on who you are and where you’re at in your career. If you have plenty of muscle to gain before you reach your genetic limit for muscle mass, you should probably consider pushing the pace (within reason). If you’ve got several years of training under your belt and believe you’re close to your genetic ceiling for muscle mass, it probably makes more sense to stay leaner and take it a bit slower (assuming you want to stay lean and don’t feel terrible at your desired body fat level). For the vast majority of lifters who have some more muscle to gain before they max out their muscular potential, fat gain should not be avoided. In fact, it should be intentionally restored to a level that allows for recovery of the endocrine system and substantial hypertrophy to occur in the offseason. This just needs to happen over a time course that could reasonably be expected to attenuate adipocyte hyperplasia, prevent fat overshooting, and allow for lean tissue restoration to occur alongside fat tissue restoration (rather than rapid and excessive fat gain, followed by slow lean tissue accretion). You could probably find enough wiggle room in the definitions of “reverse dieting” and “recovery dieting” to make this approach fit either, if you felt so inclined. A summary describing some of the factors pertaining to how quickly or slowly to increase calories is presented in Figure 12. But how fast is too fast, and how slow is too slow? That question can’t be answered without considering how long recovery is expected to take.
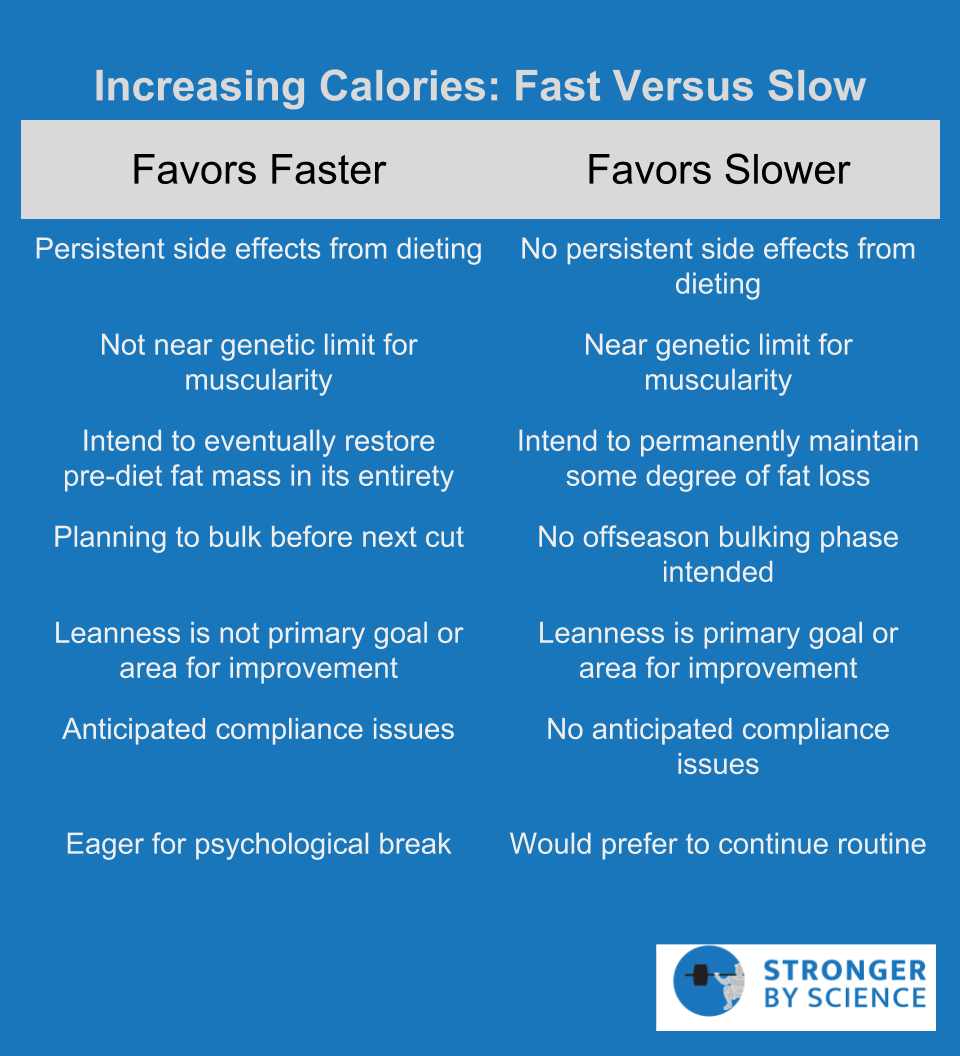
How Long Does Recovery Take?
It’s difficult to say how long it takes to recover from a diet. First of all, we need to determine what “recovery” entails. During the recovery period, we observe a reversal of adaptations related to energy expenditure, hormone levels, physical performance, reproductive function, sleep habits, and appetite, among others. The reality is that recovery is multifaceted, and different components of recovery appear to follow distinct time courses. To make things even more difficult, our behaviors and general approach to recovery make a huge impact on how quickly recovery occurs. During the recovery period, we deal with a ton of factors that are free to vary; some are under direct volitional control (resistance training load, cardio training load, energy intake, macronutrient distribution, sleep hygiene), while others are not (life stressors, heritable predispositions, psychological factors, sleep quality). When asked about the timeline for recovery, it’s tempting to say “it depends” and move on. But we might as well make some observations of the literature and do our best with interpretation.
From the case studies, we see a wide range of timelines for weight recovery; some physique athletes return to their baseline weight by nine weeks post-competition, while others are still below baseline weight a full six months after. On that topic, weight overshooting has been observed by 5-8 months post-competition, with both male and female competitors ending up a couple of kilograms heavier than they started. While the subject described by Rossow et al did not fully return to his initial bodyweight six months post-competition, he also had not yet restored his fat-free mass to baseline levels. Generally speaking, hormones more closely associated with short-term energy availability (such as ghrelin, thyroid hormone, insulin, and cortisol) start to show pretty substantial recovery in the first 3-4 months. Leptin and male testosterone levels seem to take longer; case studies measuring up to 5-6 months after competition have failed to document full testosterone and leptin recovery in males. Generally speaking, these 3-6 month hormone recovery timelines seem to fit pretty well with the most relevant study featuring a reasonably large sample. In this study of female physique athletes, 27 dieters were compared to 23 controls throughout approximately 5 months of dieting and 4 months of recovery, give or take. By the end of recovery, body composition had almost fully returned to baseline levels, and most hormones were still a little below normal, but moving in the direction of full recovery. Performance recovery seems to vary quite a bit; for example, Rossow et al described complete recovery of deadlift and squat performance within four months, but bench press remained below baseline after six months. Pardue et al reported that absolute Wingate sprint performance, assessed as both peak and average power, had increased but not fully recovered five months after competition. Using squat dynamometry, Tinsley et al showed that peak force production was modestly suppressed nine weeks after competition, but the rate of force production remained substantially below baseline.
While menstrual cycle disruption is a very common observation during preparation for physique competitions, its restoration after competition appears to be highly variable. In one case study, the participant returned to normal body weight by nine weeks post-competition, and menses resumed approximately three months after competition. In a different case study, energy availability surpassed 35 Calories per kg of fat-free mass at 10 weeks post-competition, and body weight and fat mass had been restored by week 20; nonetheless, it took a full 71 weeks of recovery for menses to resume. Regulation of the menstrual cycle is affected by a wide range of inputs, and restoration can take quite a while for many women. For example, a three-month dietary intervention successfully managed to increase energy intake and energy availability in female athletes with menstrual irregularity, but the intervention was ineffective at actually restoring regular menstruation. Other diet and exercise interventions aimed at restoring normal menstrual function have reported resumption of menses around 2-3 months and around 6-7 months after the onset of intervention. Finally, a nine-month intervention was carried out in 21 dancers and 31 athletes with menstrual disorders. After nine months, regular menses was restored in only 14.3% of the dancers and 22.6% of the athletes, and the authors speculated that the “average” time required for restoration might be upwards of a full calendar year for this sample. In this sample, restoration of menses was less likely in subjects who began the intervention with lower levels of body fat and lower energy availability; the authors speculate that increasing fat mass was a particularly important factor for resumption of regular menses. Variable recovery times are even observed in more intensive approaches; one study using exogenous leptin injections found that menstruation was restored in seven out of ten subjects, with menstruation appearing 4-32 weeks following the onset of leptin therapy.
In summary, different aspects of recovery occur over different timelines. Total energy expenditure can be partially restored in response to short-term overfeeding, but full restoration of energy expenditure seems to take longer. As positive energy balance is maintained and fat mass is restored over the first 3-6 months, hormones, energy expenditure, and performance outcomes tend to start reverting back toward baseline levels. There is a great deal of variability in recovery timelines between individuals, especially when it comes to restoring menstrual cycle irregularities. Variability is certainly related, at least in part, to how the post-diet period is approached. Recovery is likely to be accelerated in the context of a larger energy surplus, more expeditious restoration of fat mass and lean mass, more complete restoration of fat mass and lean mass, more conservative management of training loads, and more effective management of sleep and life stressors. As such, more rapid increases in caloric intake after dieting favor more rapid and complete recovery, but must be weighed against the increased likelihood of body fat overshooting. A final aspect of recovery, which may take even longer than the physiological aspects, involves psychological recovery.
Psychological Aspects After Weight Loss
Just as psychology plays an important role in weight loss, it also plays an important role in the period immediately following weight loss. The transition from dieting to maintenance or weight regain can involve shifts in motivation, perspective, habits, and body image. For this topic, I will focus on people who are dieting to unsustainable levels of leanness, such as preparation for a physique competition or photo shoot. If you’re in this position, we can make two reasonably safe assumptions: 1) you will experience noticeable fat gain in the weeks and months following weight loss, and 2) your physique is at least somewhat important to you. These types of fat loss endeavors often predispose the dieter to some rituals that probably have more negative psychological ramifications than we’d like. For example, a ton of physique athletes adhere to a morning routine in which they wake up, check their body weight for the day, and take a look in the mirror. A favorable weigh-in and a subjective improvement in the mirror can really set the tone for a positive morning during contest prep, and results from the morning weigh-in are often internalized dichotomously as “good” or “bad” progress. Luckily, an effective contest prep virtually assures incremental improvements over time, so it’s a trend over several months that has a lot more good than bad. But what happens when weigh-ins are consistently higher each day, and the mirror assessment reveals incrementally more fat? If the mind is entrained to perceive a lower weight and a leaner appearance as a “good” morning check-in, this sets the dieter up for several months of “bad” mornings. Coupled with the usual weight gain stressors (such as ever-tightening belts and clothing, a newfound accumulation of abdominal fat that becomes apparent when you sit down, or noticing that your glutes are actually covered with adipose tissue when you sit on a hard chair or bench), this can set the dieter up for a rough time.
The early weight regain phase can be associated with body image issues, a variety of disordered or problematic eating habits, reduced motivation, and the general negative affect that physique athletes call the post-competition blues. Having a plan for the period after weight loss also involves a psychological component. I’m no psychologist, so take this with a grain of salt, but it intuitively seems remarkably ill-advised to leave this component entirely unaddressed. The perspective for what constitutes a “good” check-in and a “bad” check-in should be either drastically revised, or thrown out altogether. Positive post-competition progress for a super-lean physique athlete doesn’t just allow for some fat regain, it requires it. There is tremendous benefit to embracing the positive aspects of weight regain: it’s promoting recovery of your endocrine system, it’s making you feel better on a day-to-day basis, it’s restoring your energy level, it’s giving you more dietary flexibility, it’s fueling substantially better training in the gym, and it is an instance of successfully implementing the post-competition plan. This shift in perspective has to be intentional and approached in a proactive manner, primarily to ensure that post-diet weight gain doesn’t psychologically and emotionally hit you like a ton of bricks. When psychological recovery is not proactively planned for, it can sometimes take up to 12-18 months to truly feel “normal” after a rigorous weight loss diet. When the challenges and stressors that accompany the period after weight loss are anticipated and planned for, this timeline can be dramatically shortened.
Conclusion
When you finish a weight loss diet, many of the unfavorable effects of metabolic adaptation are in full swing, and your body is primed for fat regain. If you’ve dieted to a reasonably sustainable body fat percentage, maintenance is certainly achievable. If you’ve dieted to a physique competition-level body fat percentage, weight regain is an important part of recovering from the unfavorable metabolic and hormonal effects of metabolic adaptation. However, if there’s no plan in place for transitioning from weight loss to either maintenance or a controlled weight regain process, the likelihood of excessive fat regain and body fat overshooting is increased. When the weight loss phase ends, calories should promptly be raised to maintenance levels, at minimum. Prolonging the energy deficit serves only to inhibit recovery, but the “ideal” rate at which caloric intake should increase depends on the goals and circumstances of the individual. For individuals who are comfortable returning to their initial body weight, metabolic, hormonal, and performance-related adaptations should be largely reversed within 4-6 months. Other aspects of recovery, such as restored menstrual regularity and psychological recovery, are much more variable between individuals. More conservative approaches to the post-diet recovery process that seek to delay or minimize fat regain have the potential to substantially prolong the recovery timeline.
Summary
- Weight loss attempts are met with changes in mitochondrial function, hormone levels, and energy expenditure.
- These changes produce some unpleasant side effects, threaten our ability to gain (or even keep) muscle mass and strength, manipulate biological cues pertaining to the regulation of appetite and activity level, and require us to eat even fewer calories and/or incorporate even more cardio into our training program.
- To mitigate the effects of metabolic adaptation, dieters should avoid rapid weight loss (>1% of body mass per week), eat sufficient protein (2.3-3.1 g/kg of fat-free mass), and avoid excessive cardio volumes and frequencies.
- Refeeds (twice weekly) and diet breaks (1-2 weeks at a time) may also help to attenuate the effects of metabolic adaptation.
- To avoid body fat overshooting and promote recovery, it’s important to have a transition plan in place for when the weight loss diet ends.
- The caloric deficit should not extend beyond the period of intended weight loss; the rate at which calories should be increased depends on the goals and circumstances of the individual.
- For competitors that return to (or near) their initial body weight, most physiological parameters tend to recover within 4-6 months, while menstrual cycle regularity and psychological factors are far more variable and often take longer.
- Conservative approaches that raise calories more slowly may minimize fat regain, but may also extend the timeline for full recovery.
Disclaimer: Eric Trexler is not a medical doctor or a dietitian. Speak to a qualified healthcare professional before making any changes to your diet or exercise habits.