Note: This article was the MASS Research Review cover story for October 2023 and is a review of a recent paper by Warneke et al. If you want more content like this, subscribe to MASS.
Key Points
- This review of human and animal data explored the impact of long-duration stretching on hypertrophy and strength. In animals, large and consistent increases in strength and hypertrophy occur across species with extreme (often continuous) stretching protocols.
- There are fewer human studies for ethical and logistical reasons, and they use less extreme protocols. While higher intensities, frequencies, and durations of stretching appear to cause greater gains in humans, mechanisms have only been studied in animals.
- Mechanical tension is the main proposed mechanism, but tension is multifaceted and other mechanisms may be involved. Stretch-induced hypertrophy can occur without active contraction and results in the addition of sarcomeres (in parallel and in series) in animals. It’s possible that stretching could provide a stimulus that is complementary to lifting.
When I saw this review (1), I got excited. I’ve been interested in stretch-induced hypertrophy since MASS first covered the study by Warneke and colleagues demonstrating the robust potential of long-duration, high-frequency static stretching to induce hypertrophy and strength gains in humans (2). I’ve been keeping a close eye on this research group’s work ever since. I’ve reviewed important publications like their comparison of calf stretching to calf resistance training (3), and I made a video on all the research this group published to date. Believe it or not, I’ve even gone as far as getting a similar ankle orthosis that this group used and tried it on myself for 12 weeks (we’re currently writing a case study about it).
As a huge nerd and competitive bodybuilder (and one who has struggled to make calf gains), I find this area fascinating. The big question in my mind is, how does this work? More specifically, are stretch-induced gains in hypertrophy and strength driven by the same physiological mechanisms as resistance training, or are they different? If they differ, are they potentially complementary? These are open questions, so I was excited to see Warneke and colleagues attempt to tackle some of them in the present paper, which helps us get closer to understanding what is going on with stretch-induced hypertrophy and strength gains.
Purpose and Hypotheses
Purpose
The purpose of this narrative review was “to discuss the impact of stretch-induced mechanical tension as an underlying mechanism on muscle hypertrophy and strength enhancements.”
Hypothesis
The authors did not explicitly state any hypotheses, which is common for narrative review papers.
Methods
Review Type
While narrative reviews are sometimes positioned as a lower form of evidence compared to systematic reviews and meta-analyses, many narrative reviews simply serve a different purpose. Systematic reviews and meta-analyses are best suited for topics that have a robust body of literature reporting outcome data that can be systematically gathered and summarized effectively. For this reason, they have specific guidelines for inclusion and exclusion criteria, database searching, quality control of studies, and, if a meta-analysis is performed, the statistical approach. For example, a systematic review about HMB (with or without meta analysis) would discuss all of the relevant studies exploring various outcomes of interest in various populations over the years. It would likely acknowledge the proposed mechanisms leading to those outcomes, but it should also include a qualitative or quantitative comparison of specific outcomes in response to consuming HMB versus placebos or controls.
You’ll occasionally come across a narrative review that could have been a systematic review or meta-analysis, but authors more frequently write narrative reviews to discuss a potential mechanism, cover a broad or multifaceted topic, clarify methodology, or all of the above. The present review is one such example.
Review Strategy
When writing a narrative review, researchers have a great deal of latitude when determining their search strategy and inclusion criteria. The authors described their approach as follows:
“As this is a narrative review, the authors attempted to reflect the essential state of the literature by performing an extended study search. However, because there is a vast number of studies, especially regarding the effects of stretching on flexibility in humans and on hypertrophy in animals, it was necessary to focus the literature search, which possibly led to some studies missing in the review article. To analyze studies addressing our research question, we started by screening recent systematic review articles addressing the topic (4, 5, 6, 7, 8). Subsequently, related articles and reference lists were screened to find articles excluded in the aforementioned systematic reviews. Furthermore, only studies investigating the effects of stretching on strength or strength-related parameters, such as peak torque, maximal voluntary contractions (eccentric, isometric, or concentric), or muscle mass-related parameters, were considered in this review. For a comprehensive review of the literature, systematic reviews are needed, focusing on the effects of stretching on different outcomes – flexibility, maximal strength and muscle hypertrophy – separately.”
Findings and Interpretation
As this was a narrative review without a specific qualitative or quantitative comparison, I’ll discuss their findings and my interpretations in this combined section.
Animal Findings
First, the animal data are eye-opening, for both a good and a bad reason. The good reason is the truly astounding magnitude of hypertrophy reported. The bad reason is that the protocols are extreme (as is often the case with animal research); I’m sure none of these animals enjoyed them (to skip reading about the protocols, go to the next paragraph). In most of the animal studies the researchers attached a weight or applied resistance to one wing of a chicken or a quail via a stretching apparatus. In most cases, 10-35% of the wing’s weight was used and the non-weighted wing was used as the comparator. Intermittent protocols were used in some cases (as short as two rounds of 15 minutes of stretching per day), but in most cases the wing was weighted continuously (24 hours a day) for as long as five weeks straight.
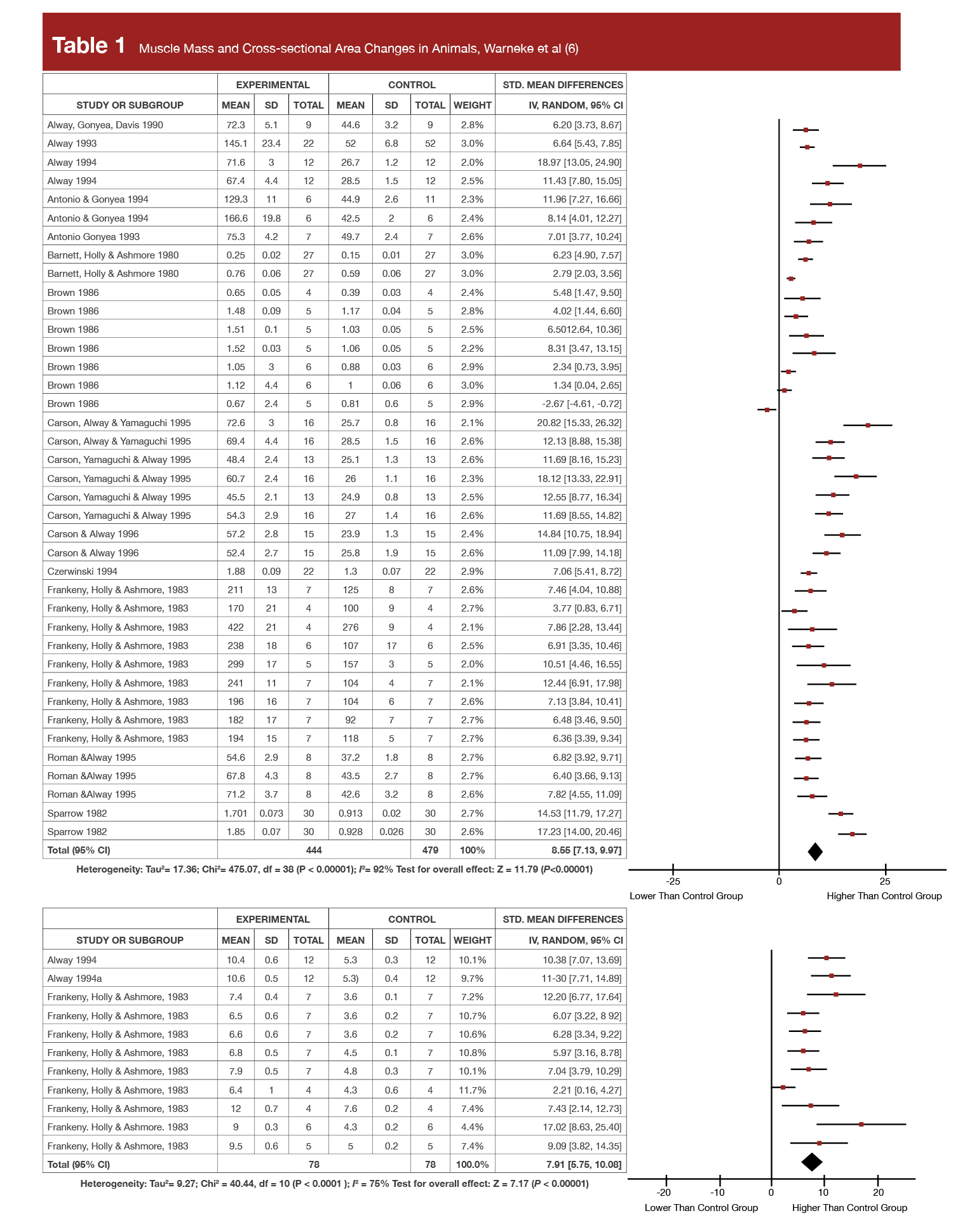
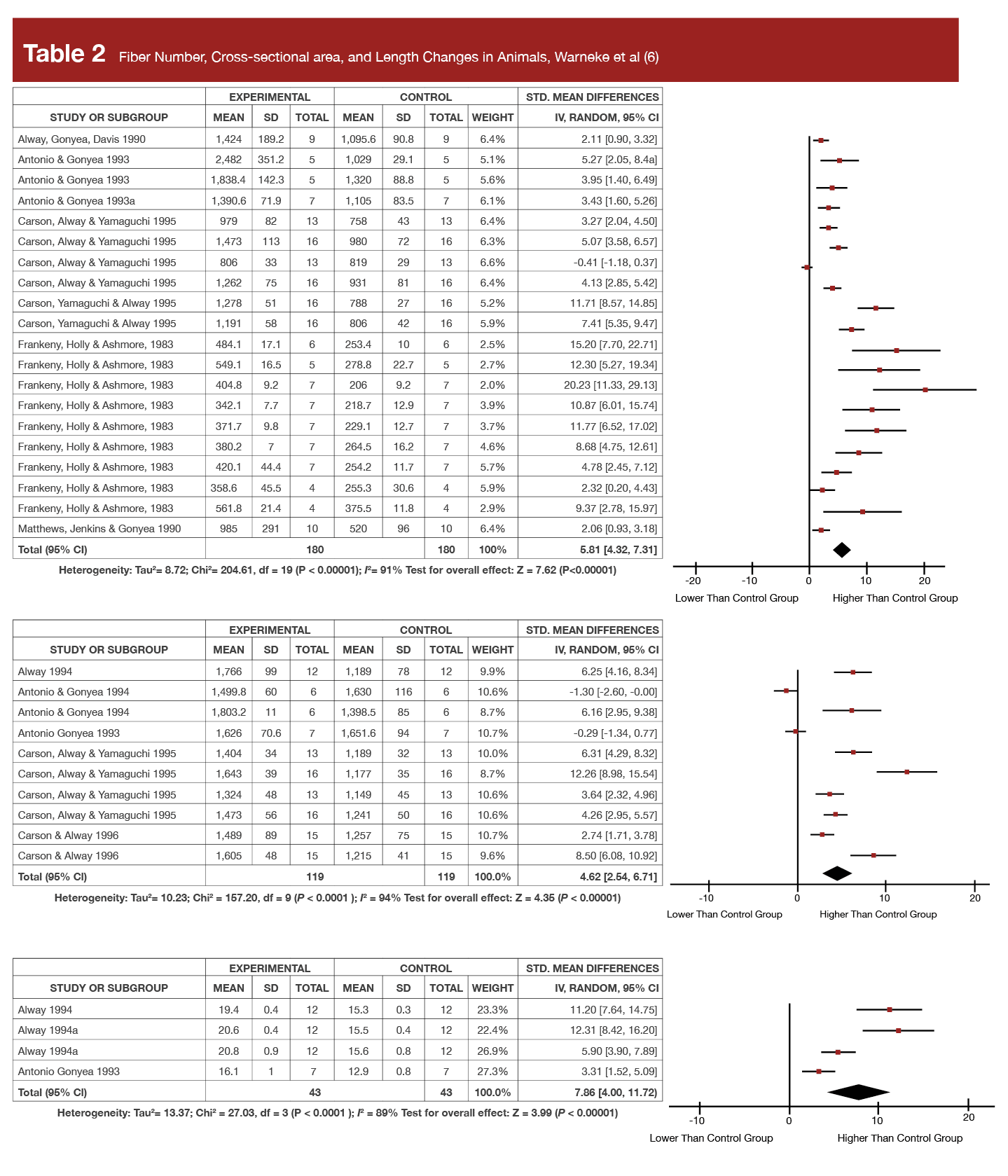
The authors reported the findings of all the animal stretching studies in the present narrative review in a really big, three-page table. But, they also published a meta-analysis on hypertrophy last year (6) on just the 16 highest quality animal studies which met their inclusion criteria, which is far easier to summarize. In that meta-analysis they reported increases in muscle fiber cross-sectional area as high as +141.6%, increases in fiber number of up to +82.2% (indicating hyperplasia), and increases in fiber length as high as +50% in chicken and quails, following interventions lasting no longer than five weeks!
To put this in perspective, Tables 1 and 2 display the forest plots from that meta-analysis for both the muscle- and fiber-level hypertrophy data showing the standardized mean changes in stretched wings relative to control wings. As a reminder, standardized mean differences are the effect size commonly reported in meta-analysis and represent proportional increases in a mean relative to their standard deviation. So, if a supplement was associated with an effect size of 0.3 for 1RM strength, the average 1RM increase after supplementation was about 30% of the pre-study standard deviation. Further, 0.2 is the threshold that researchers often use to separate a “small” effect from a trivial one, and an effect size of 0.8 or higher is typically considered “large.” You’ll see standardized mean difference values ranging from 4.62 to 8.55 in Tables 1 and 2. At first glance, it appears that stretching protocols routinely produced effect sizes 5-10x greater than the typical threshold for a “large” effect size. However, Dr. Trexler spot-checked some of the original studies to verify the effect size calculations. While he only checked a few, all of the effect sizes he spot-checked were calculated using standard error values instead of standard deviations, which dramatically inflates the effect size metric. We should take the calculated values in these tables with a big grain of salt, but the raw values and percent change values clearly tell us that the effect sizes were very substantial nonetheless (indeed, as will be discussed, the increases in muscle size are roughly 10-fold what’s been observed in humans).
While these findings are bonkers, the human data are promising but less extreme in magnitude. This is because the applicability of animal data to humans is always limited. For one, any time you compare two different species (like humans and birds) you should expect different responses because…well, they’re different species. Secondly, the ethical constraints for stretching protocols allowed in animal studies are much more liberal than the ethical constraints governing human studies. Thus, human trials simply can’t replicate the intervention or the same stimulus in most cases, and it’s also more challenging to accurately measure changes in hypertrophy (animal studies can be more precise, as the actual mass tissue is often weighted post-intervention). In the present example, both limitations apply: these birds were doing far more extreme stretching protocols than humans would ever (willingly) do.
To cross the species gap a little, we can look at the findings in non-human mammals. This area consists of just four stretching studies in rabbits and rats (9, 10, 11, 12), where like the human data, the protocols consisted of intermittent stretching. Perhaps unsurprisingly, the findings are more similar to the human data, with smaller magnitudes of change which were only observed when more frequent stretching occurred. Specifically, two of the four studies reported significant hypertrophy, one doing intermittent daily stretches in rats for three weeks (12) and the other stretching rabbits three times per week for four weeks reporting the highest muscle mass increase in non-human mammals of +13.4% (9).
Human Findings
I wanted to go over the animal data first, despite the fact that we have human data, because the findings are just so crazy. The simple magnitudes of hypertrophy reported in the animal research give reason to why this topic is worth exploring in humans. Further, as we’ll discuss in a bit, the findings also push back against some of the reductionist statements about stretch-induced hypertrophy mechanisms I’ve seen (more on that to come). This section will be brief, because we’ve already covered these data in MASS (Research Brief; video).
While the first human stretching research was conducted decades ago, the first systematic review on the topic of stretching to induce hypertrophy in humans was published in 2020 (4). Notably, the reviewed studies’ protocols were largely short-term, low-intensity, and short-duration in nature, with the longest individual stretching bouts lasting 4.5 minutes, and the highest volume being 36 minutes of stretching per week. The authors of this systematic review concluded that “passive, low-intensity stretch does not appear to confer beneficial changes in muscle size and architecture; alternatively, albeit limited evidence suggests that when stretching is done with a certain degree of tensile strain (particularly when loaded, or added between active muscle contractions) may elicit muscle hypertrophy.”
In 2022 and 2023, the first human studies using longer-duration, higher-frequency, higher-intensity protocols resembling some of the less extreme (but still effective) animal protocols were published by the authors of the present review, on the same muscle group (the calves), using the same protocol of daily stretching with a calf orthosis for an hour per day for up to six weeks in all but two cases, and at an 8/10 stretch pain tolerance. While I want to focus on mechanisms, I summarized these findings in a Volume 7 video in Issue 2 and a Research Brief in Issue 5. For convenience, I’ll restate the main findings here:
- Strength: +6.2-14.2% following 1hr/day orthosis stretching and +22.3% in the one 2hr/day study, and +10.5% in the one study using a calf board 10min/day, in six weeks.
- Muscle thickness: +4.5-15.2% following 1hr/day orthosis stretching for six weeks, with larger increases among males and in the medial versus lateral gastrocnemius.
While the data before this point were unimpressive, these findings are consistent with the non-human mammal data, reasonably impressive, and actually comparable to what’s been observed in resistance training trials of a similar length in the calves. The notable differences of the recent stretching data compared to resistance training, are for one, that unlike in resistance training trials (13), in response to stretching women seem to respond proportionally less in magnitude for both strength and hypertrophy, likely because at baseline women are more flexible on average, so the mechanical tension from stretching is lower (14). Additionally, as I discussed, 7 hours of continuous, painful stretching per week is far less logistically feasible than doing 45 minutes of total calf training per week (three days of 5 sets of 10-12 reps to failure) to get a comparable magnitude of hypertrophy or strength change (3). Thus, for most people, getting into the weeds on this topic is probably not worth it. The only reason one might consider engaging in such an extreme protocol is if they 1) had an extreme goal (say, competitive bodybuilding) and if 2) stretching turns out to be a potentially complementary stimulus to resistance training based on its mechanism(s) of action.
Mechanical Tension as a Mechanism
Now that we’ve gone through the findings, let’s talk about the proposed primary mechanism of stretch-induced hypertrophy: mechanical tension. Reading that mechanical tension is the proposed primary mechanism might have just taken the wind out of your sails if you were hoping that resistance training and stretching would have different primary mechanisms. However, mechanical tension is not just one thing. These days, “mechanical tension” gets thrown around a lot in evidence-based circles when discussing hypertrophy mechanisms, but sometimes I wonder if everyone really understands what that actually means. So, to start, let’s get into the weeds a bit on what exactly mechanical tension is.
Mechanical tension is usually induced with resistance training (but can also be induced with stretching) which stimulates the underlying cellular signaling pathways to enhance protein synthesis, resulting in muscle hypertrophy (15). This occurs through mechanotransduction, the process of an external physical stimuli (such as tension) being sensed by mechanosensors (of which there are many types in the body which sense various external stimuli) in a (muscle) cell which are then translated into biochemical signals – in this case anabolic and anti-catabolic signals which control muscle protein balance and thus, the corresponding net protein synthesis rate (16). The relevant mechanosensors to muscular adaptation are varied and located at multiple sites in skeletal muscle. Specific sensors are better placed and suited to sensing passive (stretch) and active (contractile) forces. It’s not fully clear which mechanosensors are most impacted by different training modalities (stretching versus resistance training, concentric versus eccentric muscle actions, etc.), how each mechanosensor influences cellular signaling in response to force sensing, and the degree to which each impacts downstream adaptation. With that said, a number of potential mechanosensors that may be involved in stimulating muscular adaptation in response to mechanical tension have been identified and proposed.
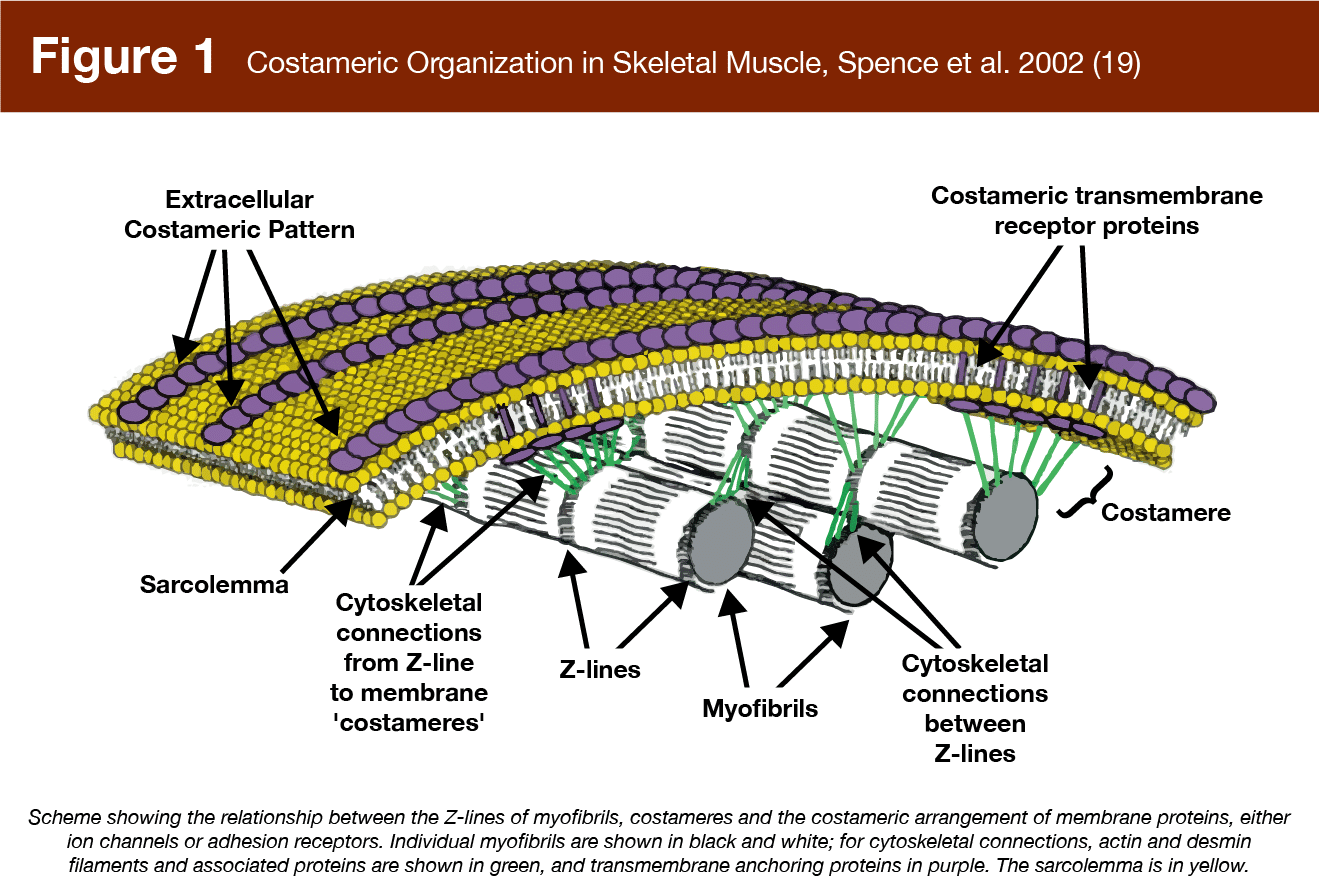
Costameres, for example (17), are protein assemblies located beneath the sarcolemma which link the extracellular matrix to the contractile component of muscle fibers (Figure 1). Costameres transmit contractile force laterally across muscle fibers to the extracellular matrix and are also proposed to sense compression forces, like muscle swelling, as well as changes in the stiffness of the extracellular matrix in response to training, potentially leading to the activation of anabolic signaling pathways (15, 18).
Titin, the largest protein in the human body, is another proposed mechanosensor with elastic properties (Figure 2). It connects the z-disk (the anchoring plate for actin filaments in the sarcomere) to the central m-line (the attachment point for myosin) and serves the functional role of providing elasticity to muscle at the sarcomere level, acting as a “molecular spring,” producing passive force when stretched (20). Titin actually goes slack during concentric contractions, but at long muscle lengths it stretches and unfolds, potentially activating anabolic signaling cascades (15).
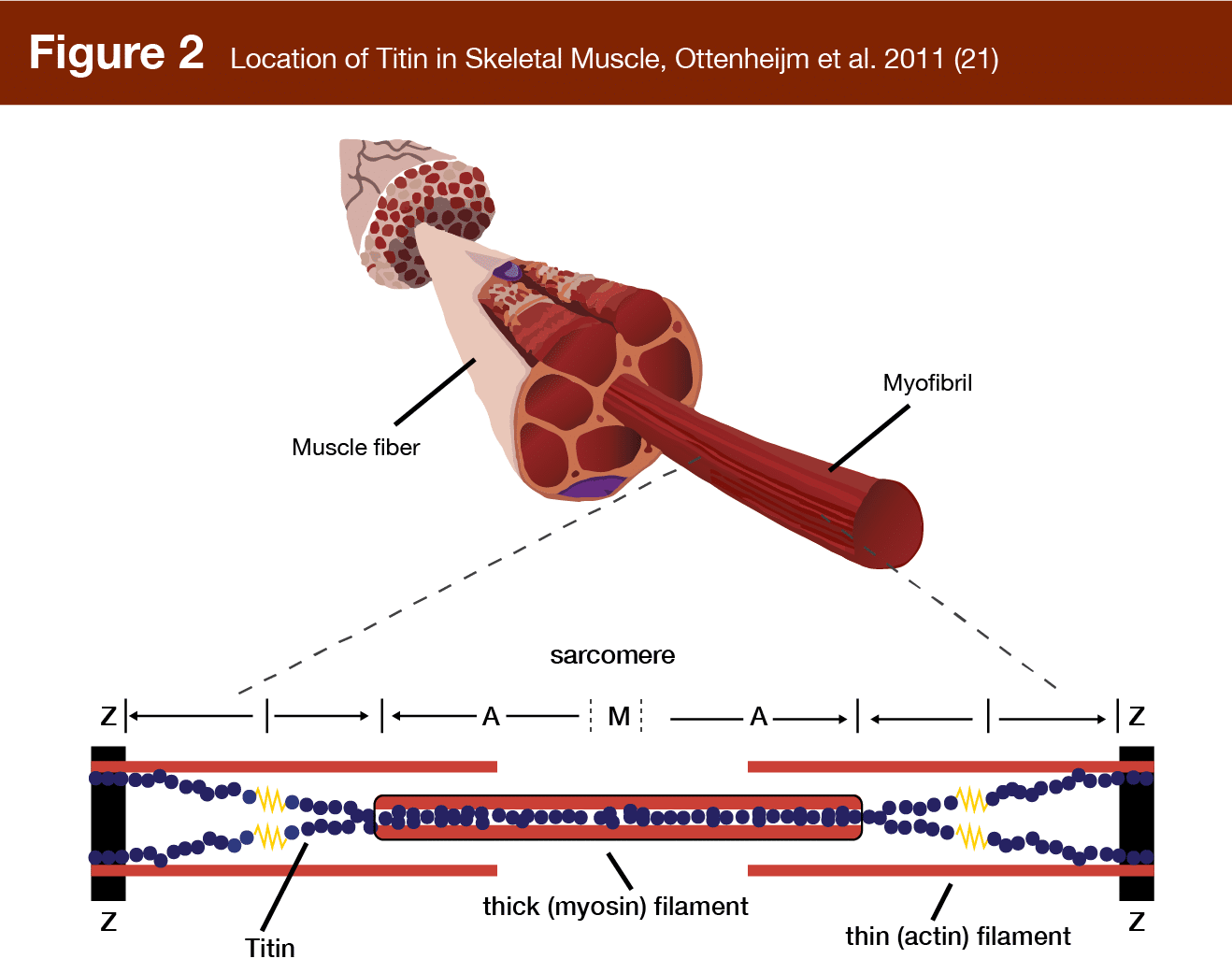
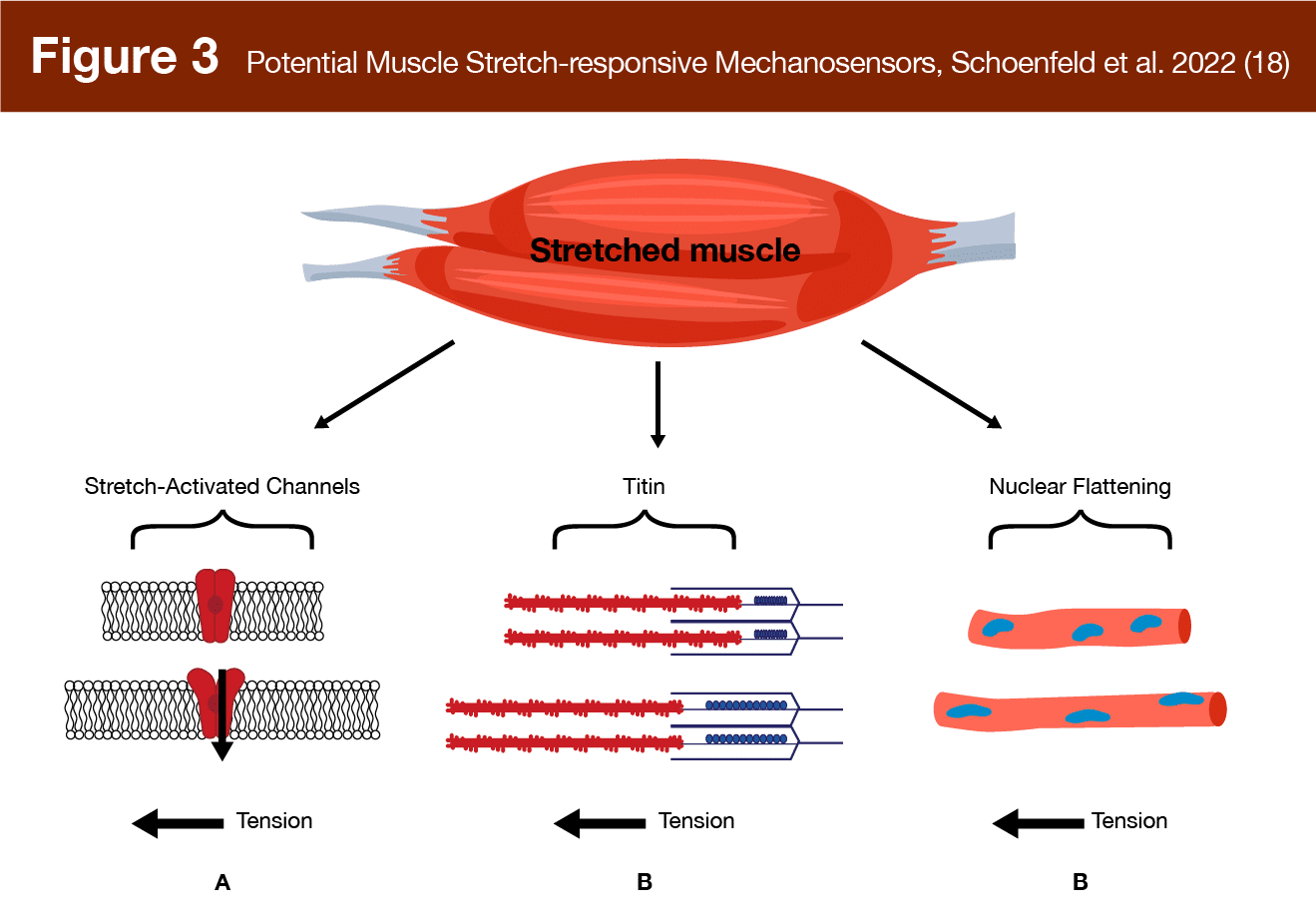
Filamins are another class of proteins that act as mechanosensors, with filamin-c specifically located at the z-disk. This v-shaped, hinge-like protein cross links actin filaments and deforms during contraction (22). Such deformation is thought to initiate signaling that could lead to hypertrophy (15). Another intriguing finding is that myonuclei themselves may act as mechanosensors (23). As myonuclei flatten and deform in response to muscle stretch, the protein desmin located beneath the sarcolemma acts as a mechanosensor detecting nuclear deformation (24), which may lead to downstream anabolic signaling (18). Finally, stretch-activated ion channels are another mechanosensor located in the membrane of muscle cells. These gate-like structures respond to stretch, which can occur due to eccentric contraction or muscle stretching, allowing ions to pass into the cell, potentially resulting in signaling cascades related to muscle damage and hypertrophy (18, 25). Thus, as shown in Figure 3, multiple mechanosensors may be able to sense and respond to muscle stretching (some of them may also sense and respond to dynamic contraction) potentially leading to hypertrophic signaling.
Once tension is sensed, hypertrophic signaling pathways are activated, several of which have been identified (e.g., mTOR/p70s6K/PI3K) and all of which are highly complex, involve anabolic and/or anti-catabolic cascades, and are likely not fully elucidated. Further, there may be other pathways (and mechanosensors) not yet identified (15, 26).
Thus, even as a primary mechanism, it’s important to understand that mechanical tension is not a singular concept. Different forms of mechanical tension can be sensed by different mechanosensors and may lead to different adaptations. Indeed, one hot take I’ve seen on the internet is that stretching will only increase the number of sarcomeres in series, but not in parallel. The claim posits that increasing sarcomeres in series is unlikely to meaningfully impact muscle thickness or cross-sectional area, thus having a minimal or negligible impact on the appearance of larger muscles. Before refuting this claim, it’s important to note that molecular-level changes (e.g., measuring sarcomere changes in parallel or series) are rarely measured in human studies and have not been specifically measured in humans in response to high-intensity, long-duration, and high-frequency stretching protocols. So, any definitive statement on this topic is arguably premature, as it’s necessarily based on animal data or indirect human data.
But, let me take a step back to ensure I didn’t lose anyone. Sarcomeres are the functional, molecular-level units of muscle fibers. Sarcomeres are where actin-myosin cross bridging occurs and force is produced. A muscle fiber is made up of thousands of sarcomeres. When a muscle fiber grows it’s largely due to the addition of more sarcomeres. Sarcomeres can be added next to one another, in parallel, or end to end, in series. Hypertrophy via the addition of sarcomeres in series is highest in response to stretch overload (as has been reported in the aforementioned animal studies) as well as eccentric training (providing overload while a muscle elongates). Importantly, muscles produce more force when more actin-myosin cross bridges can form, and thus, adding sarcomeres in series allows more cross bridges to form at longer muscle lengths (27). Therefore, the addition of sarcomeres in series is a logical, functional adaptation in response to eccentric training or stretching, as it allows the muscle to better resist elongation and produce force when in a stretched position. However, this doesn’t mean that hypertrophy in response to stretching only consists of increases in the number of sarcomeres in series. Indeed, while the muscle fiber length increases in Table 2 are huge, so are the whole muscle and individual muscle fiber cross-sectional increases in Table 1 and 2, respectively, indicating the robust addition of sarcomeres in parallel as well. Further, going all the way back to the 1970’s, there are data showing that stretching can produce hypertrophy via increases in the number of sarcomeres in series and in parallel. Intriguingly, this was documented in an animal model where the muscle was denervated (cut off from the nerve) to prevent active contraction from potentially causing the addition of sarcomeres in parallel (28).
As a final note, adaptation in the human body is often the consequence of multiple, complex, sometimes redundant, often complementary processes borne out of millions of years of evolution. While it’s psychologically satisfying to find the mechanism, more often than not there are multiple mechanisms, with one mechanism at best being primary. Indeed, in addition to the primary mechanism of mechanical tension, the authors of the present review noted that hypoxia may play a role in stretch-induced hypertrophy. In one animal study, researchers assessed microvasculature changes in rat soleus muscle in response to a stretching protocol (29). The researchers noted that stretching induced higher degrees of hypoxia and increased the number of microvascular connections and microvascular volume of the rat soleus, due to both the prolonged deformation of blood vessels during stretch, as well as ischemia (albeit to a lesser degree). The authors of the present article concluded their review by stating: “Given the different parameters influencing muscle morphology, further factors such as hypoxia, fascial tissue as well as neuronal mechanisms should be included in further research to maximize potential indicated effects.”
Next Steps
I’ve discussed the next steps regarding the applications of stretch-induced hypertrophy previously. Check out the very last paragraph here. Ultimately, we need applied research to see if stretching is additive or redundant to resistance training. However, there are different next steps if we want to uncover the underlying mechanisms as to how stretch-induced hypertrophy occurs, which would tell us if it operates differently to resistance training induced hypertrophy. Future work needs to be done specifically looking at the fiber- and molecular-level adaptations to stretching compared to resistance training. Does stretching result in conversion to slower muscle phenotypes than resistance training in humans? Does stretching increase muscle fascicle length via longer resting sarcomere lengths, or do actual increases in sarcomeres in series occur in humans? How much ischemia occurs in the stretching protocols that are feasible in humans, and how does this compare to resistance training? Further, how does it compare to BFR and can we assess how much it contributes to the observed hypertrophy? These are all open questions that a lowly applied researcher like myself can’t answer, but would love to see answered.
Application and Takeaways
If data in birds are thought to have any relevance to humans, massive hypertrophy potential is possible with long-duration, high-intensity, and high-frequency stretching. Indeed, in humans, there is a dose-response relationship between hypertrophy and strength gains and the time and intensity dedicated to stretching. The primary mechanism for these adaptations is probably mechanical tension, but not necessarily the same type of mechanical tension that occurs during resistance training. Notably, these outcomes are all reported by the same lab that has exclusively studied the calf musculature. The gains are comparable to resistance training, but may require a painful and impractical hour of daily stretching with an orthosis to achieve. For some, that might be worth it, but before you dedicate yourself to doing so, I’d advise waiting until we understand what’s going on mechanistically.
Get more articles like this
This article was the cover story for the October 2023 issue of MASS Research Review. If you’d like to read the full, 106-page October issue (and dive into the MASS archives), you can subscribe to MASS here.
Subscribers get a new edition of MASS each month. Each issue includes research review articles, video presentations, and audio summaries. PDF issues are usually around 100 pages long.
References
- Warneke K, Lohmann LH, Lima CD, Hollander K, Konrad A, Zech A, et al. Physiology of Stretch-Mediated Hypertrophy and Strength Increases: A Narrative Review. Sports Med. 2023 Aug 9.
- Warneke K, Brinkmann A, Hillebrecht M, Schiemann S. Influence of Long-Lasting Static Stretching on Maximal Strength, Muscle Thickness and Flexibility. Front Physiol. 2022 May 25;13:878955.
- Warneke K, Wirth K, Keiner M, Lohmann LH, Hillebrecht M, Brinkmann A, et al. Comparison of the effects of long-lasting static stretching and hypertrophy training on maximal strength, muscle thickness and flexibility in the plantar flexors. Eur J Appl Physiol. 2023 Aug;123(8):1773-1787.
- Nunes JP, Schoenfeld BJ, Nakamura M, Ribeiro AS, Cunha PM, Cyrino ES. Does stretch training induce muscle hypertrophy in humans? A review of the literature. Clin Physiol Funct Imaging. 2020 May;40(3):148-156.
- Kelley G. Mechanical overload and skeletal muscle fiber hyperplasia: a meta-analysis. J Appl Physiol (1985). 1996 Oct;81(4):1584-8.
- Warneke K, Freund PA, Schiemann S. Long-lasting stretching induces muscle hypertrophy: a meta-analysis of animal studies. Journal of Science in Sport and Exercise. 2022 Oct 21:1-3.
- Shrier I. Does stretching improve performance? A systematic and critical review of the literature. Clin J Sport Med. 2004 Sep;14(5):267-73.
- Medeiros DM, Lima CS. Influence of chronic stretching on muscle performance: Systematic review. Hum Mov Sci. 2017 Aug;54:220-229.
- De Jaeger D, Joumaa V, Herzog W. Intermittent stretch training of rabbit plantarflexor muscles increases soleus mass and serial sarcomere number. J Appl Physiol (1985). 2015 Jun 15;118(12):1467-73.
- Coutinho EL, Gomes AR, França CN, Oishi J, Salvini TF. Effect of passive stretching on the immobilized soleus muscle fiber morphology. Braz J Med Biol Res. 2004 Dec;37(12):1853-61.
- Gomes AR, Coutinho EL, França CN, Polonio J, Salvini TF. Effect of one stretch a week applied to the immobilized soleus muscle on rat muscle fiber morphology. Braz J Med Biol Res. 2004 Oct;37(10):1473-80.
- Coutinho EL, DeLuca C, Salvini TF, Vidal BC. Bouts of passive stretching after immobilization of the rat soleus muscle increase collagen macromolecular organization and muscle fiber area. Connect Tissue Res. 2006;47(5):278-86.
- Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005 Jun;37(6):964-72.
- Warneke K, Zech A, Wagner CM, Konrad A, Nakamura M, Keiner M, et al. Sex differences in stretch-induced hypertrophy, maximal strength and flexibility gains. Front Physiol. 2023 Jan 4;13:1078301.
- Wackerhage H, Schoenfeld BJ, Hamilton DL, Lehti M, Hulmi JJ. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J Appl Physiol (1985). 2019 Jan 1;126(1):30-43.
- Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med. 2007;37(9):737-63.
- Mathes S, Vanmunster M, Bloch W, Suhr F. Evidence for skeletal muscle fiber type-specific expressions of mechanosensors. Cell Mol Life Sci. 2019 Aug;76(15):2987-3004.
- Schoenfeld BJ, Wackerhage H, De Souza E. Inter-set stretch: A potential time-efficient strategy for enhancing skeletal muscle adaptations. Front Sports Act Living. 2022 Nov 15;4:1035190.
- Spence HJ, Chen YJ, Winder SJ. Muscular dystrophies, the cytoskeleton and cell adhesion. Bioessays. 2002 Jun;24(6):542-52.
- Krüger M, Kötter S. Titin, a Central Mediator for Hypertrophic Signaling, Exercise-Induced Mechanosignaling and Skeletal Muscle Remodeling. Front Physiol. 2016 Mar 1;7:76.
- Ottenheijm CA, van Hees HW, Heunks LM, Granzier H. Titin-based mechanosensing and signaling: role in diaphragm atrophy during unloading? Am J Physiol Lung Cell Mol Physiol. 2011 Feb;300(2):L161-6.
- Mao Z, Nakamura F. Structure and Function of Filamin C in the Muscle Z-Disc. Int J Mol Sci. 2020 Apr 13;21(8):2696.
- Aureille J, Belaadi N, Guilluy C. Mechanotransduction via the nuclear envelope: a distant reflection of the cell surface. Curr Opin Cell Biol. 2017 Feb;44:59-67.
- Palmisano MG, Bremner SN, Hornberger TA, Meyer GA, Domenighetti AA, Shah SB, et al. Skeletal muscle intermediate filaments form a stress-transmitting and stress-signaling network. J Cell Sci. 2015 Jan 15;128(2):219-24.
- McBride TA, Stockert BW, Gorin FA, Carlsen RC. Stretch-activated ion channels contribute to membrane depolarization after eccentric contractions. J Appl Physiol (1985). 2000 Jan;88(1):91-101.
- Schiaffino S, Reggiani C, Akimoto T, Blaauw B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. J Neuromuscul Dis. 2021;8(2):169-183.
- Wisdom KM, Delp SL, Kuhl E. Use it or lose it: multiscale skeletal muscle adaptation to mechanical stimuli. Biomech Model Mechanobiol. 2015 Apr;14(2):195-215.
- Sola OM, Christensen DL, Martin AW. Hypertrophy and hyperplasia of adult chicken anterior latissimus dorsi muscles following stretch with and without denervation. Exp Neurol. 1973 Oct;41(1):76-100.
- Hotta K, Behnke BJ, Arjmandi B, Ghosh P, Chen B, Brooks R, et al, Muller-Delp JM. Daily muscle stretching enhances blood flow, endothelial function, capillarity, vascular volume and connectivity in aged skeletal muscle. J Physiol. 2018 May 15;596(10):1903-1917.