Diabetes is a serious medical condition requiring ongoing management. In Type 1 diabetes, which accounts for less than 10% of diabetes cases in the United States, an autoimmune attack damages the beta cells of the pancreas, which are responsible for producing and secreting insulin when blood glucose rises. As the beta cell population becomes depleted, an individual with type 1 diabetes begins to display symptoms of insulin deficiency and impaired blood glucose regulation, which is rectified by the use of exogenous insulin. In people who are progressing toward type 2 diabetes, peripheral cells outside of the pancreas can become less responsive to insulin over time, largely influenced by factors including genetic predisposition, lack of physical activity, aging, and excess adiposity.
At first, the problem isn’t particularly severe; peripheral cells become less responsive to insulin, but our pancreatic beta cells can simply produce more insulin to induce the intended cellular response. This is known as insulin resistance, which can be characterized by high blood insulin levels, but fairly normal blood glucose levels. However, if the condition progresses, the body may become less able to effectively regulate blood glucose levels over time, leading to chronic hyperglycemia (high blood sugar) and a progressive impairment of beta cell function. As insulin resistance progresses into advanced type 2 diabetes, we increasingly see higher blood glucose levels and lower blood insulin levels, which indicate impaired glycemic control and progressive damage to beta cells.
When you have diabetes, careful management of blood glucose levels is critically important. Acute hypoglycemic episodes (low blood glucose) can induce confusion, fainting, seizures, comas, and even death (if severe and untreated). In addition, it may adversely affect brain development in children, and may cause strokes or heart attacks in older adults. On the other end of the spectrum, chronic hyperglycemia can promote progressive beta cell deterioration, atherosclerosis, cardiovascular disease, stroke, retinopathy (eye issues), nephropathy (kidney issues), and neuropathy (nerve issues). Clearly, the stakes for effective blood glucose management are high.
People with diabetes can use many tools and assessments to monitor their blood glucose regulation. For example, they may use fasted blood glucose tests, blood tests that assess their blood glucose response to a standardized meal, or blood HbA1c tests, which give a decent assessment of average blood glucose levels over the preceding 2-3 months. Another option is to use a continuous glucose monitor, which involves implanting a sensor under your skin (usually on your arm or abdomen), which continuously monitors glucose levels in your interstitial fluid (the fluid found between cells). This makes all the sense in the world for diabetes management, but you might be surprised to hear that fitness enthusiasts are increasingly using continuous glucose monitors, in hopes that they’ll provide actionable feedback for ultra-personalized nutrition strategies. Of course, in order to enable such nutrition strategies, the devices have to give glucose feedback that is both valid (accurate) and reliable (consistent).
In a recent study by Merino et al, the researchers analyzed data from a large study to assess the repeatability of continuous glucose monitor readings by looking at the level of within-device agreement (for the the Abbott Freestyle Libre Pro), and looking at the level of agreement between two different devices (the Abbott Freestyle Libre Pro and the Dexcom G6). In order to assess within-device agreement, 360 participants wore an Abbott Freestyle device on each arm. In order to assess between-device agreement, 34 participants wore the Abbott device on one arm, and the Dexcom device on the other. The available data documented blood glucose readings for 394 healthy participants, who utilized both devices over the span of 14 days in a free-living setting. Over the course of 14 days, they consumed a total of 4,457 standardized meals and 5,738 ad libitum meals.
In short, this study reported pretty solid within-device repeatability, although the agreement between devices wasn’t quite as great. For example, when looking at the coefficient of variation for 2-hour glucose area-under-curve values after standardized meals, it was 3.7% within-devices and 12.5% between-devices. In the context of ad libitum meals, values increased to 4.1% within-devices and 16.6% between-devices. Figure 1 presents the agreement between measurements from the same device model (A) and measurements from two different device models (B), along with coefficient of variation statistics (C).
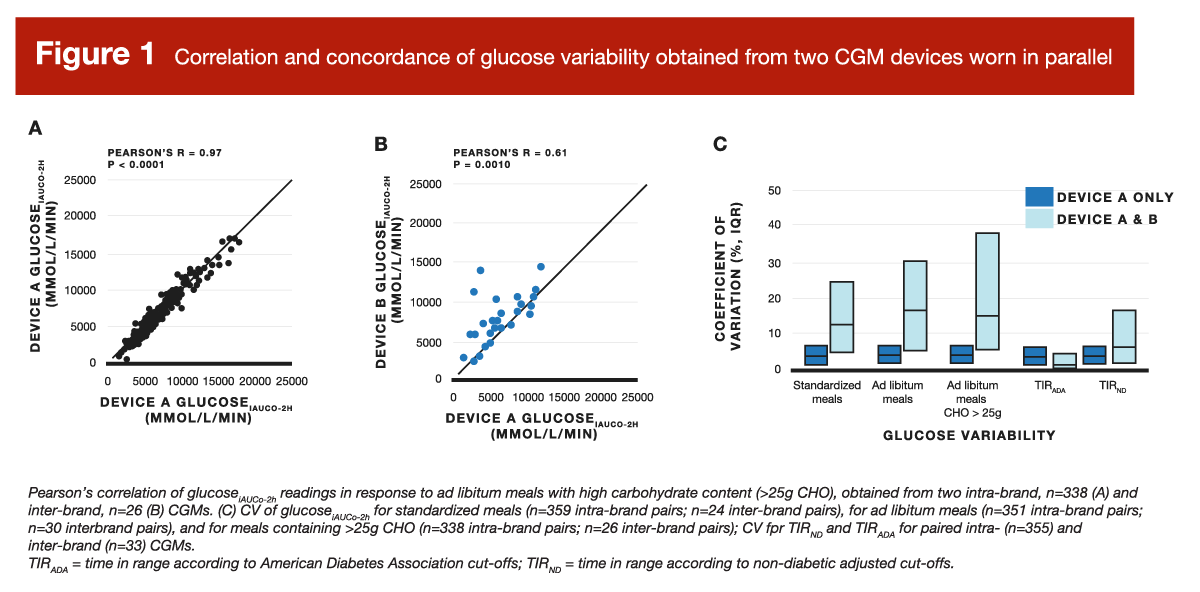
If it sounds like the science is settled and we should all be ordering some continuous glucose monitors, it’s not quite that simple. First, there are somewhat contradictory findings to consider; while the presently reviewed study reported pretty good agreement, that’s not the unanimous consensus within the literature. Howard et al reported repeatability statistics that were considerably less impressive. For example, both papers compared within-subject meal rankings (in terms of glucose responses) using the different devices. While the presently reviewed paper reported a high degree of between-device agreement (tau = 0.68), Howard et al reported considerably poorer agreement (tau = 0.43). Howard et al also reported a significant bias by which one device provided higher glucose readings than the other, but the magnitude of this bias was significantly impacted by body-fat, such that discrepancies for leaner individuals were different than those for individuals with higher body-fat levels. For a visual representation of the poor agreement reported by Howard et al, Figure 2 shows how the two devices simultaneously reported very different glucose responses to the same exact meals, eaten by the same participant.
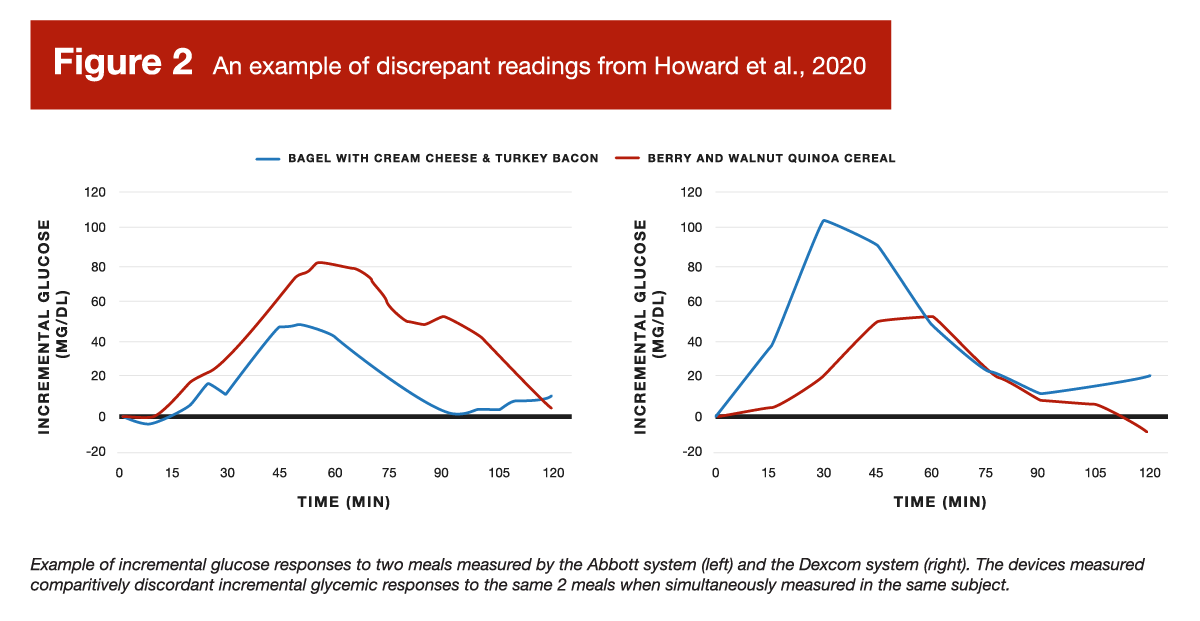
Discrepancies between the results of Howard et al and the presently reviewed study could be caused by several factors. For instance, Howard et al followed the placement recommendations provided by the device manufacturers, whereas the presently reviewed study standardized monitor placement in a manner that defied the Dexcom manufacturer instructions. While both compared an Abbott device to a Dexcom device, the specific models were different. As such, it’s possible that the newer device models used in the presently reviewed study simply perform substantially better than the older models from each company. However, even if we run with that justification, and trust that the newer models perform better, all that tells us is that these devices might help people monitor their blood glucose levels. That’s a far cry from justifying any claims about facilitating health or performance in individuals without diabetes.
I’m not fond of leaning too hard on anecdotes, but they provide great insight about the subjective experience of a particular intervention, and this particular conversation (continuous glucose monitors for health and performance in people without diabetes) is completely bereft of our scientific fundamentals in the first place. Unless I’m missing something, the purported benefits seem to be hypothetical and asserted without evidence, which means you’d be well within your “scientific rights” to reject them without evidence. However, this article from Men’s Health managed to highlight just about all of my practical concerns about continuous glucose monitors. The article contains a lot of catastrophizing about normal daily fluctuations in blood glucose, which appears to be causing unwarranted distress (“Watching my glucose level rise and fall through the day told a different, and more alarming, story than the static reading at my doctor’s office”) and orthorexic tendencies (“My [continuous glucose monitor] seemed to be okay with things like eggs and bacon in the morning, steak or grilled salmon at dinner, salads and leafy vegetables, nuts instead of chips at snack time, and pretty much anything involving avocados”).
It also led to a very predictable trend in which the devices seemed to blatantly penalize carbohydrate consumption, nudging people toward lower and lower carbohydrate intakes (“I’ve always loved carbs and have never been a fan of any kind of labeled diet, but my CGM was carb-shaming me”). If the goal is just to keep glycemic control within standard, non-diabetic ranges, then there’s no need for a continuous monitor. If, however, you use a monitor and phone app to “gamify” stability of blood glucose responses, then a ketogenic diet is a cheat code to win the game. I guess that’s fine, as low-carb and ketogenic diets are a viable dietary option, but it’s hard to frame a device as promoting hyper-individualized diet optimization for health and performance if all roads lead to carb restriction; that is explicitly not individualized, and that doesn’t jive with the evidence on high-intensity exercise performance or mortality.
In addition, the same meal eaten on multiple occasions led to very different results while using the same device (“One thing I did notice is that the various CGM sensors that I was wearing almost never agreed with one another”), and different devices provided very different feedback for the same exact meal when used simultaneously (“My glucose did not react much at all, and Levels rated the croissant a 6/10. A few days later, however, the exact same croissant from the same bakery at the same time jacked my glucose up”). This begs the question: what actionable feedback are these devices actually providing?
In the context of diabetes, it’s quite clear: my glucose is way below my intended range, so I’ll eat some carbs; my glucose is way above my intended range, so I need a larger insulin dose. If we can’t trust that our glycemic response to a meal on Monday will predict our response to the same meal ingested at the same time on Wednesday, then I reject the premise that these devices facilitate better individualized meal planning. If a glucose monitor tells us we had an unexpectedly large glucose response after a meal, we can’t confidently leverage that information for data-driven diet optimization, as we can’t be certain that our monitor will give us the same reading the next time we consume that meal. With that in mind, it seems like these devices (in their current form, when used by people without diabetes) are just quantitatively reaffirming what we already know about how glycemic responses are generally impacted by fiber, fat, glycemic load, and proximity to exercise, with some degree of variability and measurement error. I’ve seen some people suggest that they facilitate proper maintenance of carbohydrate availability during exercise, but lifters don’t have to work very hard to ensure adequate carb availability, and “bonking” due to low carb availability during endurance exercise is an easily identifiable and unmistakable sensation (no sensors or phone apps needed).
Biohacking and physiological data monitoring are all the rage right now, so I totally understand why people are so interested in continuous glucose monitors. If that’s your hobby and it brings you joy, then go for it – trying to minimize post-meal glucose excursions will do no physiological harm, and I have no reason to rain on your parade. However, I would respectfully push back at anyone who confidently asserts that this intervention is entirely harmless, or carries no risks of any kind. When we consider psychological aspects of diet and exercise habits, there is potential harm in convincing people that normal post-meal increases in blood glucose are to be feared. There is potential harm in baselessly reinforcing the idea that carbs are inherently deleterious. There is potential harm in convincing people to manage a very scary problem they didn’t know they had, and that the best way to manage it is by adopting an unnecessarily and unsustainably rigid approach to food selection.
So, if continuous glucose monitors excite you, I’m not here to deprive you of a fun experiment. However, if I were concerned about my insulin sensitivity, I’d personally focus on increasing my physical activity level, losing some fat mass, and adjusting my daily fiber, protein, and calorie targets as needed. If I was having hypoglycemic episodes or suspected that I was trending toward pre-diabetes or diabetes, I’d simply pay a visit to a qualified medical professional to discuss treatment options. In that scenario, it’s possible that a continuous glucose monitor would be recommended. However, for non-clinical applications related to general health or performance in people without glycemic control issues, I’m not seeing much evidence to support a beneficial effect, and there are some potential downsides when we burden ourselves with the task of micromanaging glucose fluctuations that are well within normal ranges.
Note: This article was published in partnership with MASS Research Review. Full versions of Research Spotlight breakdowns are originally published in MASS Research Review. Subscribe to MASS to get a monthly publication with breakdowns of recent exercise and nutrition studies.




