Note: This article was the MASS Research Review cover story for May 2023 and is a review of a recent paper by Smith et al. If you want more content like this, subscribe to MASS.
Key Points
- Changes in satellite cell number and ribosome content after 10 weeks of resistance training, along with acute myofibrillar protein synthesis in response to a single exercise bout, were assessed in 34 untrained young women to determine the extent to which these outcomes were predictive of muscle hypertrophy in higher and lower responders.
- There was no significant association between the change in vastus lateralis cross-sectional area and myofibrillar protein synthesis (r = 0.095; p = 0.602) or changes in muscle ribosome content (r = 0.014; p = 0.937). Satellite cell number increased significantly in higher responders (p = 0.026), but not lower responders (p = 0.118), and the percent change in satellite cells per fiber was correlated with the mean change in fiber cross-sectional area (r = 0.471; p = 0.007).
- Although the authors report that satellite cell abundance was more reflective of the resistance training response than muscle ribosome content, they acknowledge that this is inconsistent with previous data, and that several methodological limitations preclude their ability to discount a role for ribosome biogenesis in the hypertrophic response.
Skeletal muscle hypertrophy involves an increase in the diameter of individual muscle fibers, which results in an increase in total cross-sectional area. Hypertrophy occurs when the rate of muscle protein synthesis exceeds that of muscle protein breakdown. Protein synthesis requires the following steps:
- In a process known as transcription, sections of DNA are copied to form three types of RNA: messengerRNA (mRNA), transferRNA (tRNA) and ribosomalRNA (rRNA). Think of mRNA as the template, or the set of instructions, for protein synthesis. rRNA and ribosomal proteins form the building site and tRNA transports materials to the site.
- The next step is known as translation, in which tRNA attaches to specific amino acids according to the mRNA instructions, and brings them to the ribosomes where proteins are synthesized. You might have heard the term “ribosome biogenesis,” which is the process of making new ribosomes. The translational capacity of the muscle fiber is dependent on the number of available ribosomes; a building site is required to build new proteins. In this sense, muscle protein synthesis and ribosome content are inextricably linked.
The mRNA, or “instructions,” are copied from DNA in the nucleus. As a muscle fiber grows, its ability to keep up with the transcriptional and translational requirements becomes more limited (3). In this scenario, satellite cells (muscle stem cells) can assist by donating nuclei, adding to the “machinery” available for translation, and, thus, increasing translational capacity.
There is compelling evidence to demonstrate “higher” and “lower” hypertrophic responders to resistance training. The underlying factors may include differences in the untrained state, including genetics, satellite cell number, and ribosome content (2). In some cases, high responders appear to experience a greater increase in muscle protein synthesis and ribosome content in response to resistance training, resulting in a larger increase in muscle size compared to that of low responders (2). The differential response to training may include the increase in myonuclei or remodeling of the extracellular matrix due to satellite cell proliferation (2).
These physiological differences between higher and lower responders have been explored in men, and it is unclear if there could be sex-based differences. Thus, Smith and colleagues assessed protein synthesis, changes in satellite cell number, and changes in ribosome content in young, untrained women (1). This was a secondary analysis of a subset of participants in a study from the same lab group that investigated the influence of peanut protein supplementation on strength and hypertrophic outcomes (4). The purpose of the analysis, which included 34 untrained young women, was to determine the extent to which the following outcomes were predictive of muscle hypertrophy, and if they differed between higher and lower responders:
- Acute myofibrillar protein synthesis following an exercise bout
- Chronic changes in satellite cell number in response to resistance training
- Changes in ribosome content in response to resistance training
The researchers assessed the 24-hour myofibrillar protein synthetic response to a single exercise session, which consisted of three-repetition maximum (RM) leg press, barbell bench press, and hex-bar deadlift strength assessments, followed by two sets of 10 repetitions of each exercise at 50% of the participants’ estimated 1RM. Then the participants completed supervised resistance training sessions twice per week (one higher load session and one lower load session) for 10 weeks. The exercises included the leg press, barbell bench press, knee extension, hex bar deadlift, and lat pull down. The higher load sessions consisted of five sets of 6 repetitions, while the lower load sessions consisted of four sets of 10 repetitions.
Higher and lower responders were classified using a composite change score (from baseline to post-training) of the following variables: Dual-energy X-ray absorptiometry (DXA) lean/soft tissue mass, vastus lateralis cross-sectional area (measured with ultrasound), mid-thigh muscle cross-sectional area (measured with peripheral quantitative computed tomography), and 3RM hex-bar deadlift strength (Figure 1). Based on the composite scores of the 34 participants, the eight individuals in the upper quartile were labeled “higher responders” and the eight individuals in the lower quartile were labeled “lower responders.” The authors in the presently reviewed study assert that an inclusive, multidimensional approach to delineating responder sub-groups should include strength and hypertrophy measures. It’s worth acknowledging that statistically quantifying true response heterogeneity is quite difficult, and there is much disagreement about how to properly categorize responders.
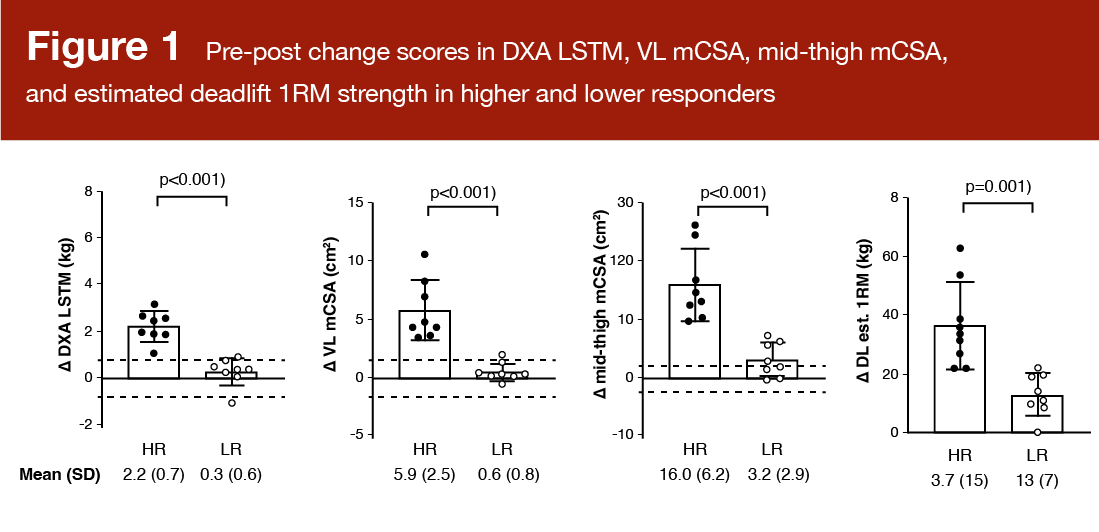
There were no differences between higher and lower responders in the 24-hour myofibrillar protein synthetic response to the exercise bout at baseline. The average rate was 2.25 ± 1.05% per day (HR: 1.88 ± 0.98% per day; LR: 2.43 ± 1.25% per day). There was no significant association between myofibrillar protein synthesis and the change in vastus lateralis cross-sectional area (r = 0.095; p = 0.602). The researchers were not surprised by these findings, particularly because they only assessed a 24-hour period, and the exercise bout was a novel, likely damaging stimulus.
There was no difference in RNA content per mg of wet muscle from baseline to post-training in any of the participants, and there was no significant correlation between changes in muscle ribosome content and the change in vastus lateralis cross-sectional area (r = 0.014; p = 0.937). This finding is not consistent with previous data in untrained men (2), which points to changes in ribosome content as a distinguishing factor between higher and lower responders. While RNA concentration, or ribosome density, is reflective of ribosome biogenesis, this measurement is just a snapshot at one time point, and doesn’t give us a complete picture of a very dynamic process. In any case, it would be, in my opinion, highly premature to highlight this as a known sex-based difference without a lot more research.
Satellite cell number increased significantly from baseline to post-training in higher responders (p = 0.026), but not lower responders (p = 0.118). There was a significant positive correlation (r = 0.471; p = 0.007) between the percent change in satellite cells per fiber and the mean change in fiber cross-sectional area. These results are shown in Figures 2 and 3.
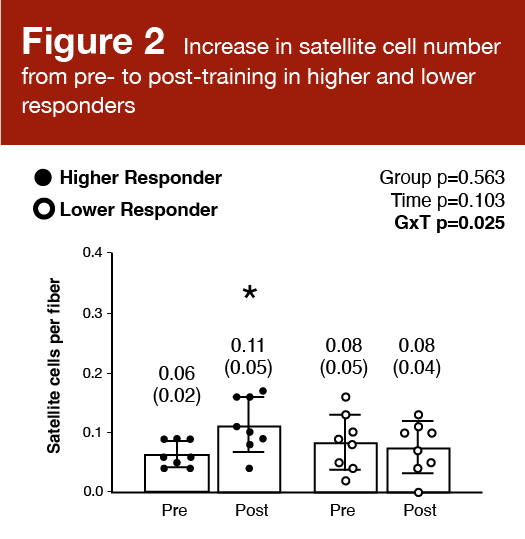
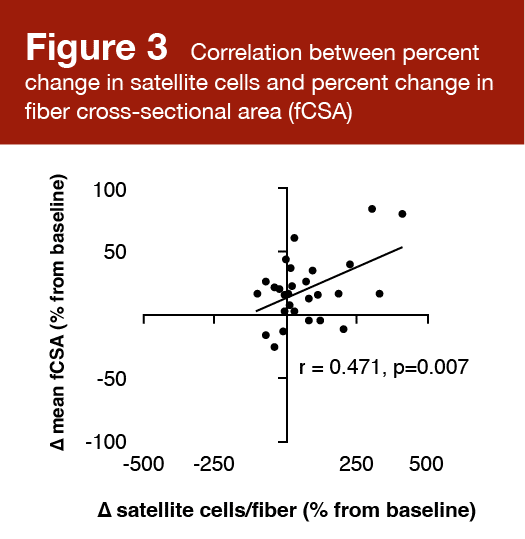
These findings align with other data suggesting that myonuclear addition drives superior growth (5), and that the number of satellite cells in an untrained individual is a determinant of his/her hypertrophic potential (6). That said, the extent to which satellite cells are required for hypertrophy is heavily debated. Satellite cells can repair or replace damaged muscle fibers or divide and self-renew to maintain the stem cell pool. An increase in muscle fiber size is often associated with an increase in the number of myonuclei. Satellite cell differentiation results in fusing and donating nuclei to an existing fiber. There is some evidence to suggest that the proliferation, differentiation, and fusion of satellite cells are required for hypertrophic adaptations in both young and older individuals (7) that occur with a concomitant increase in fiber cross-sectional area (8). An “extreme” growth response to resistance exercise appears to include a large increase in the satellite cell pool. The simplest evidence to support the contribution of satellite cells to muscle fiber growth is an evaluation of long-term anabolic steroid users. Eriksson and colleagues reported vastly larger muscle fibers and a greater number of myonuclei per fiber in these individuals (9). There was a positive correlation between the number of myonuclei per fiber and the fiber cross-sectional area, which provides compelling evidence that myonuclear number contributes to hypertrophic adaptations.
That said, satellite cells certainly serve other functions, including remodeling of the satellite cell niche and maintenance/renewal of the satellite cell pool. Thus, an increase in satellite cell number is not necessarily indicative of a growth response (10). This is further demonstrated by evidence that satellite cells contribute to non-hypertrophic remodeling in response to aerobic interval training (11). It is conceivable that satellite cells are nonessential for muscle hypertrophy in the short term, assuming an upregulation of transcriptional capacity by existing myonuclei. However, they are likely required for longer-term growth and the maintenance of muscle mass, tissue health, and function.
Application and Takeaways
This body of literature doesn’t provide very practical take-home messages for the coach or the lifter. However, it gives us some insight into the complex process of muscle hypertrophy, which is pretty fascinating in my opinion! It is also a reminder of the variability in the response to resistance training between individuals. Perhaps at some point in the future, we will be able to tease out the mechanisms for higher and lower responses to particular resistance training programs. For example, why do some individuals respond better to higher volume or higher intensity training than other individuals? There is a lot more work to do in this area, and our ability to explore these nuanced, complex processes will improve with the development of more sensitive analytical techniques.
References
- Smith MA, Sexton CL, Smith KA, Osburn SC, Godwin JS, Beausejour JP, Ruple BA, Goodlett MD, Edison JL, Fruge AD, Robinson AT, Gladden LB, Young KC, Roberts MD. Molecular predictors of resistance training outcomes in young untrained female adults. J Appl Physiol (1985). 2023 Mar;134(3):491-507.
- Roberts MD, Haun CT, Mobley CB, Mumford PW, Romero MA, Roberson PA, Vann CG, McCarthy JJ. Physiological Differences Between Low Versus High Skeletal Muscle Hypertrophic Responders to Resistance Exercise Training: Current Perspectives and Future Research Directions. Front Physiol. 2018 Jul 4;9:834.
- Brook MS, Wilkinson DJ, Smith K, Atherton PJ. It’s not just about protein turnover: the role of ribosomal biogenesis and satellite cells in the regulation of skeletal muscle hypertrophy. Eur J Sport Sci. 2019 Aug;19(7):952-963.
- Sexton CL, Smith MA, Smith KS, Osburn SC, Godwin JS, Ruple BA, Hendricks AM, Mobley CB, Goodlett MD, Frugé AD, Young KC, Roberts MD. Effects of Peanut Protein Supplementation on Resistance Training Adaptations in Younger Adults. Nutrients. 2021 Nov 9;13(11):3981.
- Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006 Nov;291(5):E937-46.
- Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985). 2008 Jun;104(6):1736-42.
- Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2001 Jun;56(6):B240-7.
- Blocquiaux S, Gorski T, Van Roie E, Ramaekers M, Van Thienen R, Nielens H, Delecluse C, De Bock K, Thomis M. The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Exp Gerontol. 2020 May;133:110860.
- Eriksson A, Kadi F, Malm C, Thornell LE. Skeletal muscle morphology in power-lifters with and without anabolic steroids. Histochem Cell Biol. 2005 Aug;124(2):167-75
- Mackey AL, Holm L, Reitelseder S, Pedersen TG, Doessing S, Kadi F, Kjaer M. Myogenic response of human skeletal muscle to 12 weeks of resistance training at light loading intensity. Scand J Med Sci Sports. 2011 Dec;21(6):773-82.
- Joanisse S, Gillen JB, Bellamy LM, McKay BR, Tarnopolsky MA, Gibala MJ, Parise G. Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J. 2013 Nov;27(11):4596-605




