This article is a review and breakdown of a recent study. The study reviewed is Muscle Fiber Hypertrophy in Response to 6 Weeks of High-Volume Resistance Training in Trained Young Men is Largely Attributed to Sarcoplasmic Hypertrophy by Haun et al. (2019)
Key Points
- In a reanalysis of data from a prior study, it was found that the subjects who had meaningful increases in muscle fiber cross-sectional area also tended to have decreases in actin and myosin (contractile protein) concentrations, and thus a relative increase in the proportion of the muscle fiber composed of sarcoplasm.
- In other words, this study provides solid evidence of sarcoplasmic hypertrophy.
- What causes sarcoplasmic hypertrophy? How might we train to attain it (or avoid it)? Those are still open questions, but we’re at the point where we can make some educated guesses.
Sarcoplasmic hypertrophy was a bogeyman of the evidence-based fitness world for years. Considered the stuff of muscle mag legend, it was long disregarded with little more than a scoff. However, a recent study (1) provided further compelling evidence for the existence of the phenomenon. I say “further” evidence, because the earliest study demonstrating sarcoplasmic hypertrophy is 50 years old, but the good references were so few and far between that it wasn’t taken seriously.
Just to back up, what does “sarcoplasmic hypertrophy” mean? To keep things simple, your muscle fibers have loads of structures called myofibrils, which are primarily composed of the contractile proteins actin and myosin. The rest of the stuff inside the muscle fiber is called the sarcoplasm, which is composed of organelles, proteins, glycogen, water, and a bunch of other various non-contractile elements. When a fiber grows, it’s generally assumed that the proportion of the fiber composed of myofibrils either stays the same or increases; that would be called “myofibrillar hypertrophy.” If, on the other hand, the fiber grows, but the proportion of the fiber composed of myofibrils decreases, that means the sarcoplasm has expanded at a greater rate than the myofibril pool; that’s sarcoplasmic hypertrophy.
In the presently reviewed study (1), 15 men who had robust increases in muscle fiber cross-sectional area following a high-volume six-week training program were analyzed. The density of contractile proteins (actin and myosin) in their muscle fibers decreased, indicating that sarcoplasmic hypertrophy had occurred. So, why did this occur? What implications does this have for designing training programs? There are a lot of open questions, but I think we can make some educated guesses. Read on to learn more.
This article is from a previous issue of Monthly Applications in Strength Sport (MASS), our monthly research review with Greg Nuckols, Eric Trexler, Eric Helms and Mike Zourdos. Every month, we analyze 10 of the most important studies for strength and physique athletes and coaches, then write about the practical applications in concise, jargon-free research reviews (like this one!).
If you're interested in staying up to date with the latest strength and nutrition research, you can find the MASS subscription plan that works for you and subscribe here.
Purpose and Hypotheses
Purpose
The purpose of this study was to investigate the changes in mitochondrial volume and contractile protein, sarcoplasmic protein, and glycogen concentrations that occur with hypertrophy.
Hypotheses
The authors hypothesized that the subjects would experience a decrease in muscle actin and myosin concentrations, a decrease in citrate synthase activity (suggesting decreased mitochondrial density), and either no change or an increase in glycogen and sarcoplasmic protein concentrations.
Subjects and Methods
Subjects
This study is a continuation of the work done in a previous study (2), which was thoroughly explained here by the study’s lead author. The subjects in that previous study were 31 young men with at least one year of training experience, and a minimum squat of 1.5 times body mass. In the presently reviewed study, the subjects were the 15 people from the previous study who had notable (>320μm2) increases in muscle fiber cross-sectional area.
Study Design
In the initial study (2), the training program consisted of three training sessions per week for six weeks, consisting of five different exercises. This study was based primarily on data gathered from vastus lateralis (lateral quad) biopsies, so for our purposes here, we’ll just focus on the study’s squat training. Throughout the study, the squat training consisted of sets of 10 with 60% of 1RM. On week 1, the subjects performed 10 total sets of squats (4 on day 1, 2 on day 2, and 4 on day 3). Volume increased each week, so by week 6, they were performing 32 sets of squats (12 on day 1, 8 on day 2, and 12 on day 3).
The researchers took vastus lateralis biopsies and blood draws prior to the start of the training protocol, after three weeks of training, and after six weeks of training. The biopsies took place 24 hours after the last training session on weeks 3 and 6, so to examine the effects of recovery following the training program, a subsample of seven participants also had biopsies taken seven days after their week 6 biopsy. During the eight days between the end of the training program and the final biopsy, those seven subjects were asked to refrain from all lifting.
The variables the researchers examined were total levels of actin and myosin (your two primary contractile proteins) in the muscle fibers, density of actin and myosin, glycogen concentrations, citrate synthase activity (as a marker for mitochondrial volume), several markers of muscle damage and protein breakdown, and changes in sarcoplasmic proteins.
Prefer to listen? Check out the audio roundtable
Every article we review in MASS has an accompanying audio roundtable just like this one. All of the MASS reviewers (Greg Nuckols, Eric Trexler, Eric Helms, and Mike Zourdos) get together to discuss the findings and applications in practical, easy-to-understand terms. Subscribe to MASS here.
Findings
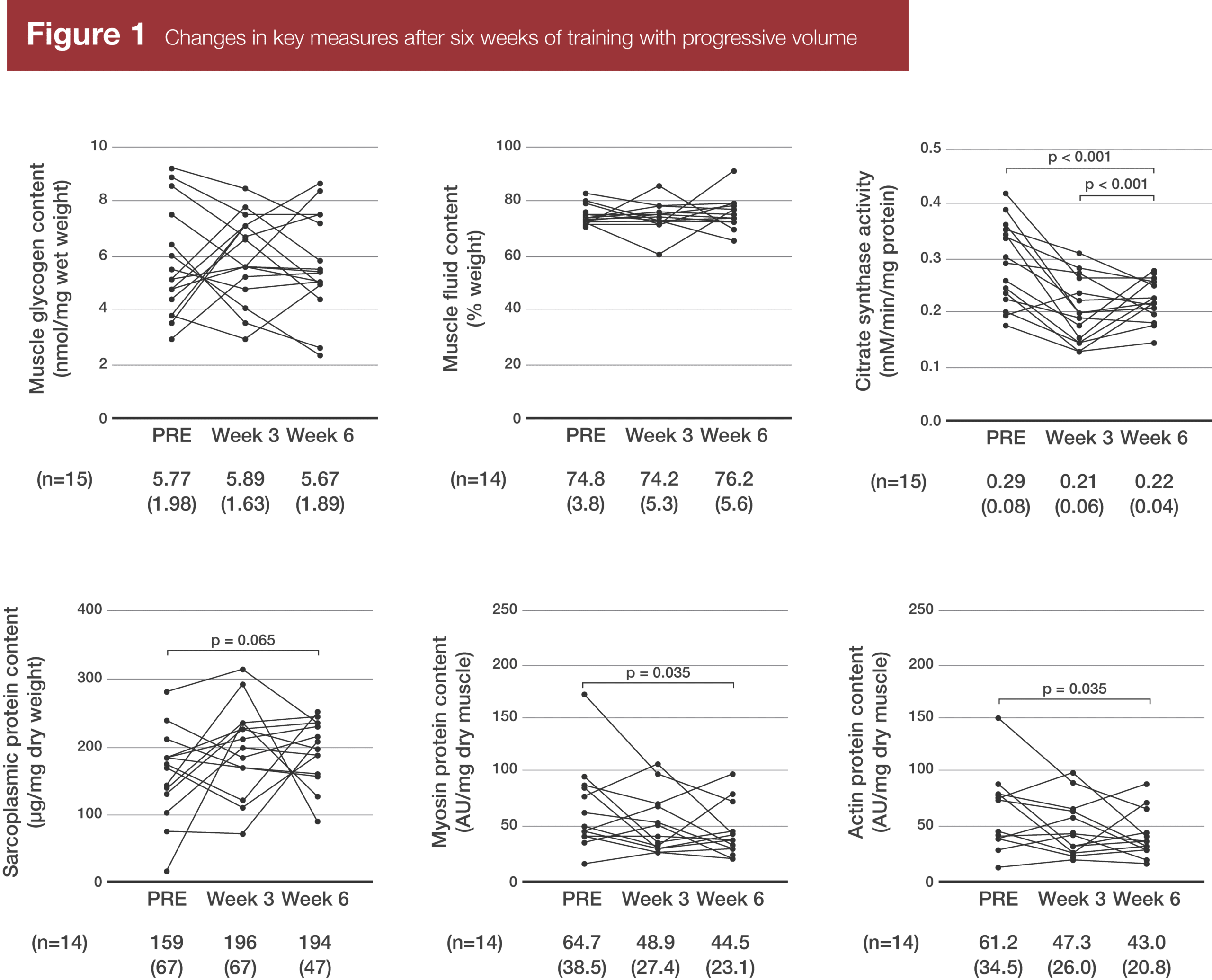
Glycogen concentrations, fluid content, and sarcoplasmic protein concentrations didn’t significantly change from pre-training to post-training. Myosin and actin concentrations, on the other hand, significantly decreased (p = 0.035) from pre- to post-training, as did citrate synthase activity. Furthermore, while the change in sarcoplasmic protein concentration wasn’t significant, it did tend to increase (the average change was about +23% and the p-value for the pre- to post- change was 0.065), but it was quite variable.
To be clear, contractile protein content didn’t decrease. In chemically stained fibers, the amount of actin per fiber didn’t significantly change. However, no change in actin coupled with an increase in fiber size led to a decrease in actin concentrations. Also worth noting, pre-training and after three weeks of training, total actin content was closely associated with fiber cross-sectional area (r2 = 0.815 pre-training and 0.867 after three weeks of training). However, post-training, there was no longer a significant relationship between actin content and fiber cross-sectional area (r2 = 0.160; p = 0.22).
In spite of the mean decrease in actin and myosin concentrations, markers of protein breakdown (20S proteasome activity and ubiquitinated protein levels) and muscle damage (serum creatine kinase activity) didn’t significantly change over the course of the study. Just eyeballing the graphs, creatine kinase levels may have trended up over the course of the study, but the change for each individual was highly variable, so the changes weren’t statistically significant.
Although total sarcoplasmic protein concentrations didn’t increase, certain sarcoplasmic protein levels did increase, including those associated with glycolysis and gluconeogenesis.
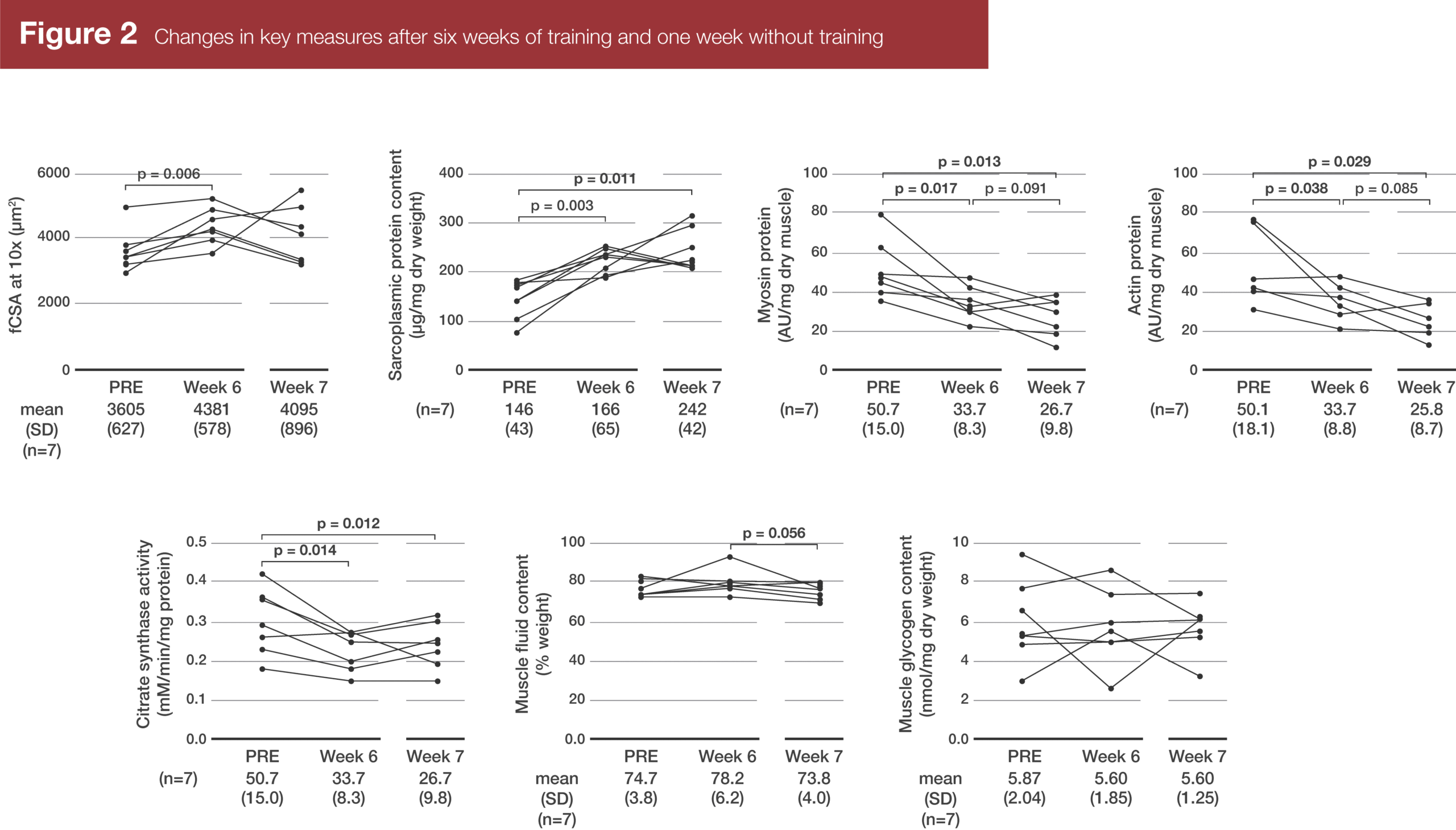
In the subsample of seven participants who were biopsied again eight days after the final training session, fiber cross-sectional area decreased back toward baseline, such that it wasn’t significantly different from either the pre-training or post-training fiber sizes. Unlike the full sample of 15 subjects, sarcoplasmic protein content had significantly increased from pre-training to post-training in this subsample, and it tended to increase further after a week of rest. Complementarily, actin and myosin concentrations tended to decrease from week 6 to week 7.
Interpretation
Just as a disclosure before I get rolling on this interpretation: The lead author of this study (Cody Haun) is a coach for Stronger By Science and has previously contributed to MASS. I don’t think that influenced my selection or interpretation of this study, but it is a relationship you, dear reader, may appreciate being informed of.
There are two ways to interpret the findings of this study. The first, more conservative approach is to be pretty skeptical of these findings. There was one subject with exceptionally high actin and myosin concentrations pre-training (>150 AU/mg, when all other subjects were <100 AU/mg), and his actin and myosin concentrations decreased precipitously during the first three weeks of training. Excluding that one subject who looks like a bit of an outlier, the decreases in actin and myosin concentrations were no longer statistically significant (p = 0.053, up from p = 0.035). If that one subject is simply a gross abnormality, then we may be getting excited about sarcoplasmic hypertrophy, when in reality we’re looking at something much more mundane: a false positive in small sample research.
The second, more liberal approach is to tentatively take the findings at face value. That’s what I’m inclined to do here. If this was the first study suggesting the existence of sarcoplasmic hypertrophy, I’d be a lot more skeptical. However, sarcoplasmic hypertrophy has shown up in the literature several times before, dating back at least 50 years, so it’s not a shocking result, at least if you’re up-to-date on the research (3, 4, 5, 6, 7). As such, I see the barely significant and not-quite-significant results of the present study as a simple indication of relatively low statistical power (which is understandable, given the time and monetary cost of the biochemical analyses performed in this study).
So, sarcoplasmic hypertrophy can definitely occur; we can confidently make that statement based not just on this study, but on multiple prior studies with similar observations. That leaves us with a few interesting questions:
- What is its purpose?
- How common is it?
- Do we know how to influence it with training?
The purpose of sarcoplasmic hypertrophy
Several years ago, I wrote an article about sarcoplasmic hypertrophy, in which I proposed that increases in proteins associated with anaerobic metabolism were the most likely driver of the phenomenon. The reasoning was pretty straightforward: lifting is metabolically costly, but aerobic metabolism gets less efficient as muscle fibers get larger (especially if you’re not doing dedicated aerobic training) since mitochondrial density often decreases, and the diffusion distances for oxygen entering the muscle fiber increase as fiber cross-sectional area increases. Therefore, as muscle fibers grow, they’ll become more reliant on anaerobic metabolism, they’ll produce more proteins associated with anaerobic metabolism, and those proteins will pull water into the muscle fiber, expanding the sarcoplasm.
The findings of this study largely support that hypothesis. Citrate synthase activity did decrease, suggesting a decrease in mitochondrial density, and sarcoplasmic protein content tended to increase (p = 0.07; d = 0.5). Of the specific sarcoplasmic proteins that increased in concentration, many are involved in anaerobic metabolism (glycolysis and gluconeogenesis).
I admit that I may simply want to take these results at face value because they support my biases, but I think they’re logical findings. For most tissues in your body, their top hardwired goal is maintaining energy homeostasis. When you train, especially if it’s metabolically taxing training, the threat to energy homeostasis would be interpreted as a bigger threat than the threat of the tension itself. Therefore, expanding the pool of proteins involved in rapid ATP production is the first priority, with increases in contractile tissue coming later. That’s the model that the authors propose (personal correspondence) – initial dilution (but not loss) of contractile protein as proteins involved in anaerobic metabolism (and possibly proteins associated with the T-tubules and sarcoplasmic reticulum as well) increase in concentration, followed eventually by concomitant increases in contractile protein once the metabolic stage has been set to deal with more metabolic stress (which would result from gaining more contractile proteins, getting stronger, and thus being able to expose your muscles to higher workloads).
I think that’s a very elegant model, as it accounts for the function of sarcoplasmic hypertrophy. One of the reasons a lot of people are skeptical of the existence of sarcoplasmic hypertrophy is that they think of it as “nonfunctional” hypertrophy, because it’s unlikely to increase muscular strength. Since it’s illogical for muscles to adapt to training in a completely nonfunctional manner, thinking of sarcoplasmic hypertrophy as “nonfunctional” hypertrophy does make sarcoplasmic hypertrophy seem unlikely and illogical. But once you realize that it does likely have a purpose – a metabolic purpose rather than a purpose predicated on force output – its existence becomes a lot more logical and palatable.
How common is sarcoplasmic hypertrophy?
Unfortunately, most labs aren’t as well-equipped as the one where this study was carried out, so few papers directly assess changes in myofibrillar density (and, by extension, sarcoplasmic hypertrophy). However, we can get some clues from studies that assess functional characteristics of individual muscle fibers. The force a muscle fiber can produce per unit of cross-sectional area (also known as “specific tension”) should scale linearly with its myofibrillar protein density. So, if we see single-fiber force output increasing more than fiber cross-sectional area, we can assume that myofibrillar density is increasing, and if we see single-fiber force output increasing slower than fiber cross-sectional area, we can assume that myofibrillar density is decreasing.
According to a recent meta-analysis from Dankel et al (8), fiber specific tension generally increases with training, meaning force output increases faster than cross-sectional area. For type I fibers, across 15 studies, force increased by 17.5% while cross-sectional area increased by 6.7%, while for type IIa fibers, across 14 studies, force increased by 17.7%, while cross-sectional area increased by 12.1% (though it’s worth noting that this wasn’t a significant difference; however, the two realistic options based off the nominal differences are an increase in specific tension or no change in specific tension, but a decrease doesn’t seem to be realistic). So, it’s clear across multiple studies that sarcoplasmic hypertrophy can occur, but it’s also clear that most research finds that a disproportionate increase in myofibrillar protein density is actually more likely with training.
Do we know how to influence sarcoplasmic hypertrophy with training?
At this point, I think the conservative answer is “no.” However, we do have some clues. If sarcoplasmic hypertrophy is predominantly driven by increases in proteins involved in anaerobic metabolism, then more metabolically stressful training should cause more sarcoplasmic hypertrophy as an adaptive response. In other words, a high volume of sets of 8+ reps would potentially do the trick. For example, the present study used an extremely high volume of sets of 10, and caused a fair amount of sarcoplasmic hypertrophy. Contrast those results with this study where the subjects were doing a moderate volume of sets of 4-6 reps, where almost all of the participants had an increase in myofibrillar protein density (9); additionally, a single set to failure at 90% of 1RM failed to increase sarcoplasmic protein synthesis 24 hours post-training, while training to failure at 30% of 1RM did increase sarcoplasmic protein synthesis for at least 24 hours post-training (10). In a recent meta-analysis (11), “high load” training (mostly in the 8-12 reps range) and low load training (mostly in the 20-30 rep range) were found to lead to similar increases in both muscle size and isometric force, suggesting that the ratio of myofibrillar to sarcoplasmic protein accretion was probably similar between moderate rep and high rep training, which is what makes me think that, contrary to popular opinion, it’s not just very high rep training that can cause sarcoplasmic hypertrophy. Sarcoplasmic hypertrophy may be less likely with low rep training (<5-8 reps per set), and more likely with both moderate (8-15 reps per set) and high rep (15+ reps per set) training. And on the topic of set volume, these two studies (12, 13) involved quad training at a similar intensity, and this one (12) found that 10 sets did not increase sarcoplasmic protein synthesis beyond what could be elicited from protein feeding, while this study (13) found that 20 sets did cause a robust increase in sarcoplasmic protein synthesis. So, both doing more reps per set and doing more total sets may increase the likelihood of sarcoplasmic hypertrophy.
Another factor that may matter is training status. In the present study (1), the lifters were pretty well-trained, and they experienced sarcoplasmic hypertrophy. Most of the studies in the Dankel et al meta-analysis (8), on the other hand, used untrained lifters, and the results suggest that disproportionate myofibrillar hypertrophy occurred (increased specific tension). Furthermore, in this study (4), myofibrillar volume density was lower in a sample of well-trained bodybuilders and powerlifters than untrained controls, and in this study (14), the specific tension of bodybuilders’ muscle fibers was considerably lower than controls’. Thus, all else being equal, myofibrillar hypertrophy may predominate early in a training career, and sarcoplasmic hypertrophy may play an increasing role as training status increases. That would make sense if we assume that sarcoplasmic hypertrophy is an adaptation to help deal with increased anaerobic metabolism – since the energy cost of training scales with work rate (force × distance / time), as lifters get progressively stronger, the anaerobic demands of training should increase, and as lifters’ muscle fibers get progressively larger, aerobic metabolism should become increasingly less efficient, necessitating greater contributions from anaerobic energy systems.
General thoughts
In this review, I’ve expressed a lot of opinions and ideas, but I want to reiterate that these are just my best guesses. The entire idea of sarcoplasmic hypertrophy was overlooked in the literature for a long time, so for the time being, all we can do is attempt to make the best inferences possible with a small body of direct research, plus a bit of indirect research.
This does raise some interesting questions, though. For example, it’s generally thought that low rep training (sets of 5 or fewer reps) is less efficient than moderate or high rep training for building muscle on a per-set basis. However, it’s possible that myofibrillar protein accretion is similar all the way down to heavy doubles or triples (though I do still doubt that heavy singles are the bee’s knees for muscle growth), but total change in muscle size is just smaller with low rep sets because less sarcoplasmic expansion occurs. We also don’t know if the authors’ idea that sarcoplasmic hypertrophy “sets the stage” for myofibrillar hypertrophy is accurate or not; if it is, then it may be something worth pursuing for virtually all lifters. If it’s not, and the two processes are totally separate, then strength athletes may want to purposefully minimize sarcoplasmic hypertrophy (once we know what exactly causes it) since, presumably, it would increase the lifter’s mass without helping them lift more. We also don’t know if there’s a limit to the amount of sarcoplasmic hypertrophy that can take place; maybe it’s decoupled from myofibrillar hypertrophy, so you can achieve enormous amounts of sarcoplasmic expansion with relatively little myofibrillar protein accretion, and keep growing for years. On the other hand, there may be mechanisms that keep sarcoplasmic hypertrophy from getting out of control, tying the limits of sarcoplasmic expansion to limits of myofibrillar protein accretion (in which case, you’d want to primarily focus on myofibrillar hypertrophy for long-term gains). We also need more direct research into the sorts of training that are more or less likely to cause preferential sarcoplasmic hypertrophy.
Finally, I’d just like to highlight how variable the baselines and responses were in the present study. Pre-training, myosin concentrations varied from about 17 AU/mg, all the way up to about 170 AU/mg. That’s a 10-fold difference in contractile protein density! And the changes in myofibrillar protein density ranged from a 59% decrease to a 58% increase. Kudos to the researchers for providing figures that show individual responses, along with providing their raw data. In MASS, we also want to make it clear that group averages don’t always apply to individuals. Your contractile protein density and how much sarcoplasmic hypertrophy you experience after really high volume training are variables that this principle extends to.
Next Steps
We need studies to directly assess what style of training is most likely to promote sarcoplasmic hypertrophy. I’d propose a training study with four groups: (1) one group doing a moderate amount of sets of 5, (2) one group doing a moderate amount of sets of 10, (3) one group doing twice the volume of sets of 5, and (4) one group doing twice the volume of sets of 10. I’d hypothesize that group 1 would experience little to no sarcoplasmic hypertrophy, groups 2 and 3 would both experience sarcoplasmic hypertrophy to similar degrees, and group 4 would experience the most sarcoplasmic hypertrophy.
Application and Takeaways
- Sarcoplasmic hypertrophy can absolutely occur. It is not a myth. I repeat, it is not a myth.
- The amount of sarcoplasmic hypertrophy you experience may depend on the rep range you train in (with more occurring with sets of 8-10+ or more reps), and the set volume you train with (with more occurring with higher set volumes). It may also depend on training age, with more experienced lifters experiencing more sarcoplasmic hypertrophy. This takeaway depends on the assumption that sarcoplasmic hypertrophy primarily occurs to fuel increased anaerobic metabolism.
- We need a lot more research to fill in all the details.
The latest research – interpreted and delivered every month
If you want to stay up-to-date on the research pertinent to strength and physique athletes and coaches, but you don't have the time or desire to develop the skill set to critically analyze research, you can sign up for Monthly Applications in Strength Sport (MASS), the monthly research review I put out every month, along with Dr. Eric Trexler, Dr. Eric Helms, and Dr. Mike Zourdos.
Each issue of MASS contains at least 10 pieces of content like this. Click here to learn more and join 3,250+ subscribers.
References
- Haun CT, Vann CG, Osburn SC, Mumford PW, Roberson PA, Romero MA, Fox CD, Johnson CA, Parry HA, Kavazis AN, Moon JR, Badisa VLD, Mwashote BM, Ibeanusi V, Young KC, Roberts MD. Muscle fiber hypertrophy in response to 6 weeks of high-volume resistance training in trained young men is largely attributed to sarcoplasmic hypertrophy. PLoS One. 2019 Jun 5;14(6):e0215267.
- Haun CT, Vann CG, Mobley CB, Roberson PA, Osburn SC, Holmes HM, Mumford PM, Romero MA, Young KC, Moon JR, Gladden LB, Arnold RD, Israetel MA, Kirby AN, Roberts MD. Effects of Graded Whey Supplementation During Extreme-Volume Resistance Training. Front Nutr. 2018 Sep 11;5:84.
- Penman KA. Ultrastructural changes in human striated muscle using three methods of training. Res Q. 1969 Dec;40(4):764-72.
- MacDougall JD, Sale DG, Elder GC, Sutton JR. Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. Eur J Appl Physiol Occup Physiol. 1982;48(1):117-26.
- Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, Palmer BM. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol. 2012 Mar 1;590(5):1243-59.
- Roberts MD, Romero MA, Mobley CB, Mumford PW, Roberson PA, Haun CT, Vann CG, Osburn SC, Holmes HH, Greer RA, Lockwood CM, Parry HA, Kavazis AN. Skeletal muscle mitochondrial volume and myozenin-1 protein differences exist between high versus low anabolic responders to resistance training. PeerJ. 2018 Jul 27;6:e5338.
- Ribeiro AS, Avelar A, Schoenfeld BJ, Ritti Dias RM, Altimari LR, Cyrino ES. Resistance training promotes increase in intracellular hydration in men and women. Eur J Sport Sci. 2014;14(6):578-85.
- Dankel SJ, Kang M, Abe T, Loenneke JP. Resistance training induced changes in strength and specific force at the fiber and whole muscle level: a meta-analysis. Eur J Appl Physiol. 2019 Jan;119(1):265-278.
- Cribb PJ, Hayes A. Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Med Sci Sports Exerc. 2006 Nov;38(11):1918-25.
- Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010 Aug 9;5(8):e12033.
- Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW. Strength and Hypertrophy Adaptations Between Low- vs. High-Load Resistance Training: A Systematic Review and Meta-analysis. J Strength Cond Res. 2017 Dec;31(12):3508-3523.
- Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009 Feb 15;587(Pt 4):897-904.
- Louis M, Poortmans JR, Francaux M, Berré J, Boisseau N, Brassine E, Cuthbertson DJ, Smith K, Babraj JA, Waddell T, Rennie MJ. No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol Endocrinol Metab. 2003 Nov;285(5):E1089-94.
- Meijer JP, Jaspers RT, Rittweger J, Seynnes OR, Kamandulis S, Brazaitis M, Skurvydas A, Pišot R, Šimunič B, Narici MV, Degens H. Single muscle fibre contractile properties differ between body-builders, power athletes and control subjects. Exp Physiol. 2015 Nov;100(11):1331-41.