A while ago, we published an article in the MASS Research Review that questioned the popular belief that high body-fat levels impair p-ratios. Then we followed up with an expanded and more in-depth Stronger By Science article on the topic, and Menno Henselmans published a rebuttal on his site shortly thereafter. We responded to his rebuttal, and shortly after our rebuttal was posted, Menno added to his article to respond to it. Unfortunately we feel the need to respond yet again, as his response lacked substance and was pretty misleading. Since this has already been a pretty protracted back-and-forth, we’re going to assume you’re already up to speed, and dive straight in.
Menno states: “Regarding the rodent study, the fact is the researchers reported the high-fat diet group gained the most weight and fat, and this was the group that gained the least muscle afterwards. That’s proof of principle that a ‘dreamerbulk’ can interfere with subsequent muscle growth. While their final body-fat percentage may not have ended up considerably higher than the other group, they were the only group that developed insulin resistance, which brings us to an important point.
“Trexler & Nuckols focus intensely on exact body-fat percentages and the exact correlations of it, but this is partly missing the point. Even the bro theory of nutrient partitioning acknowledges body-fat percentage per se is not the problem: insulin resistance is (plausibly via the associated inflammation). In practice we can’t directly observe inflammation or insulin resistance or whatever obesity-related problems may interfere with muscle growth, so the idea is that we should stay away from body-fat ranges that may cause these problems. BF% is thus just a proxy for the real problem(s).”
This is pretty wild. The first paragraph of the claim essentially asks us to accept the fact that two groups got similarly obese, only one of them experienced impaired hypertrophy, but this is somehow proof of principle that your body-fat level should be kept low in order to avoid hypertrophy impairment. In reality, this provides a direct counterpoint to his claim that body-fat percentage should be kept low to avoid hypertrophy impairment. The only way to make this argument logically consistent is to acknowledge that high body-fat is not the factor driving hypertrophy impairment, despite the fact that Menno’s specific recommendation is to keep body-fat low, not to manage insulin resistance or inflammation at your current level of body-fat.
In Menno’s original rebuttal to our argument, one of the few things we agreed on was that insulin resistance does not directly impair p-ratios. Now he appears to be suggesting that insulin resistance is a necessary prerequisite for body-fat to interfere with hypertrophy, and that obesity in the absence of insulin resistance shouldn’t be a problem at all. This is somewhat reminiscent of our contention that body-fat alone doesn’t seem to be a major impediment to hypertrophy, especially among people who are exercising, given that exercise attenuates most of the scary mechanisms that are used to justify the old p-ratio hypothesis. The premise of using small gradations in body-fat percentage as a proxy for multifactorial outcomes like insulin resistance or inflammation, then extrapolating from there to make inferences about hypertrophy potential, is totally unsubstantiated.
As a reminder, the mice who got to 46.6% body-fat in this study had excellent hypertrophy outcomes, and the mice who got to 48.1% body-fat had markedly impaired hypertrophy compared to the “normal-weight” mice around 38.8-39.1% body-fat. Now, do we have solid evidence that a group of clinically obese people with impaired insulin sensitivity can achieve great hypertrophy, even while losing body-fat? Absolutely; it was in our original argument, when we showed that American football linemen tend to be obese and insulin resistant but tend to make great gains (and leaner gains) than their leaner, more insulin-sensitive counterparts. But why should we let robust human data from healthy, resistance trained young men get in the way of a great narrative?
It seems like quite a stretch to state that this study is “proof of principle” that gaining substantial amounts of fat independently interferes with hypertrophy. Two groups gained fat and ended up at approximately the same body-fat percentage; one had blunted hypertrophy, while the other achieved the second-greatest hypertrophy out of five groups. Now, is there any other difference between those two rodent groups that might explain differential effects on hypertrophy, aside from the <2% gap in their body-fat percentages? Absolutely; they were consuming different diets with different energy densities, food sources, and macronutrient ratios. Those seem like they could be a lot more impactful than a negligible difference in body-fat percentage, which is why the study authors confidently concluded that the dietary differences were most likely to be driving the divergent effects.
It should also be noted that one of the core contentions in our initial rebuttal was that exercise attenuates most of the mechanisms one could point to in an effort to link higher body-fat levels to impaired hypertrophy. In the rodent study, however, hypertrophy was not induced by exercise per se. Functional overload was induced via synergist ablation: the soleus and gastrocnemius of the mice were removed to make the plantaris the sole plantar flexor, leading to overload of the plantaris during normal ambulation. As such, the rodent study likely doesn’t provide a great proxy for the effects of body-fat on resistance training-induced hypertrophy (synergist ablation isn’t really “exercise”; it just makes day-to-day life more challenging for the target muscle). If anything, it suggests that, even independent of exercise (and its capacity to mitigate mechanisms by which higher body-fat levels may interfere with hypertrophy), different body-fat levels don’t have a large independent effect on hypertrophy. At this point, though, it’s probably not worth focusing on the rodent study anymore; Eric really only used it initially as an excuse to discuss the broader topic in MASS, and given the fact that there’s relevant human research on the topic, it’s not central to our case.
Menno’s comment also asserts that we can’t directly observe inflammation or insulin resistance in practice, but we can’t figure out what that means. There are numerous biomarkers for each, which can easily be assessed at just about any doctor’s office or laboratory. His argument also suggests that we are “missing the point” by focusing on body-fat per se rather than the consequences of high body-fat (such as insulin resistance), which may or may not be present when body-fat gets higher. Yet, Menno’s central conclusion is focused purely on keeping body-fat low rather than the management of insulin sensitivity or inflammation (which can be influenced by numerous factors other than adiposity), within an arbitrary body-fat range that is based on no direct evidence at all.
Menno states: “Regarding inflammation, we seem to agree excess inflammation is clearly bad for our gains. Trexler & Nuckols argue that it’s just unlikely to ever become a problem for an overall healthy strength trainee, because all the research is on older adults. That’s certainly a notable limitation, and it’s safe to say any negative effects would be smaller at lower levels of inflammation, but would they disappear completely? We actually have a study on young, healthy strength trainees that found a negative correlation between a marker of inflammation, baseline Il-6, and muscle growth, but Trexler & Nuckols disregard this due to lack of data reporting.”
This doesn’t respond to any of the substantive arguments we made, which cast major doubts on Menno’s application of this concept. If body-fat is an important driver of hypertrophy-blunting inflammation, why did none of the studies he cites report that body-fat was predictive of gains, or that high-inflammation groups had markedly higher body-fat than low-inflammation groups? If baseline IL-6 is the important factor to consider, and he cited three hypertrophy studies that actually measured baseline IL-6, why did two (one, two) of the three find no significant relationship between resting IL-6 and hypertrophy? Even if you simply defer to the consensus of the few studies Menno presents, they actually lean against his argument. In the study that did find a relationship between resting IL-6 and blunted hypertrophy (but did not measure body-fat), how do we know IL-6 wasn’t elevated due to the long list of factors that can influence inflammation levels independent of body-fat (like stress, sleep, sedentary time, and diet), which were totally uncontrolled and unaccounted for? Also, why were other biomarkers of inflammation (CRP and TNF-alpha) unrelated to hypertrophy in the very same study?
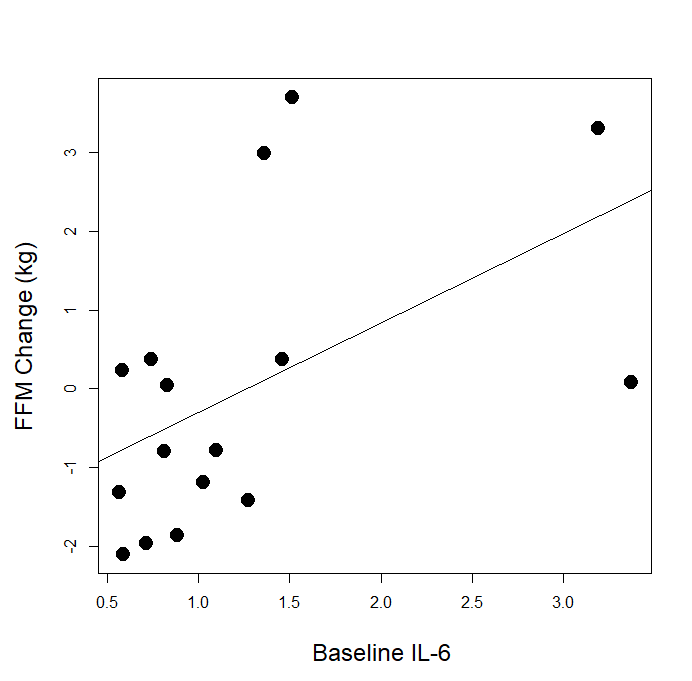
It seems that Menno is comfortable with a huge proportion of his argument leaning fully on the IL-6 results from Mitchell et al, who reported a negative correlation (for which the strength of the correlation and the p-value were unreported) between baseline IL-6 and hypertrophy. It’s worth noting that correlations in small samples are notoriously unstable, and that you should always be cautious about putting too much stock in the results from a single study. It’s also worth noting that IL-6 data were made available to us for the study by Klemp et al, and baseline IL-6 was positively correlated with hypertrophy (r = 0.52, p = 0.040).
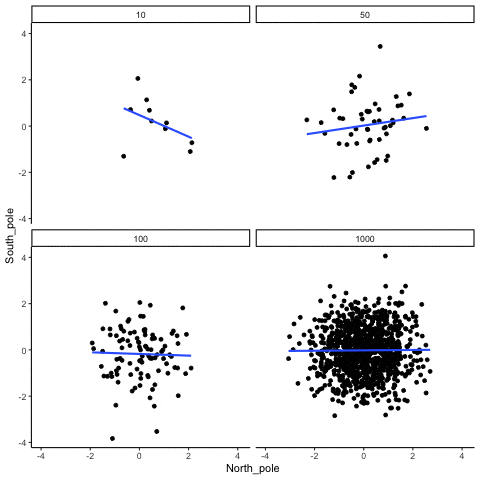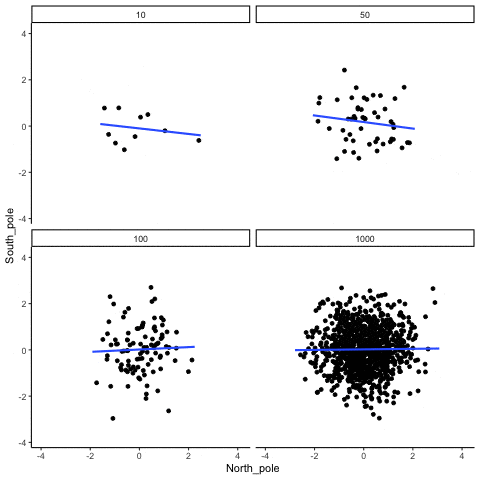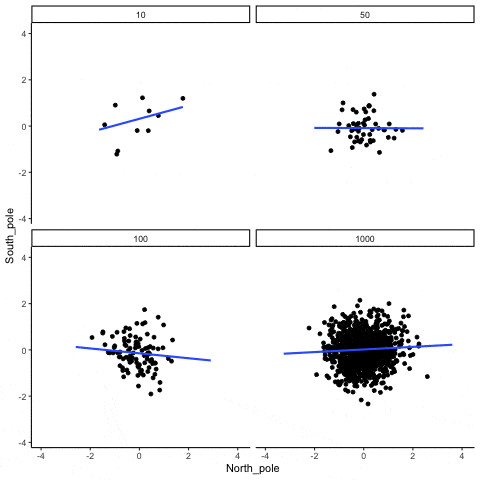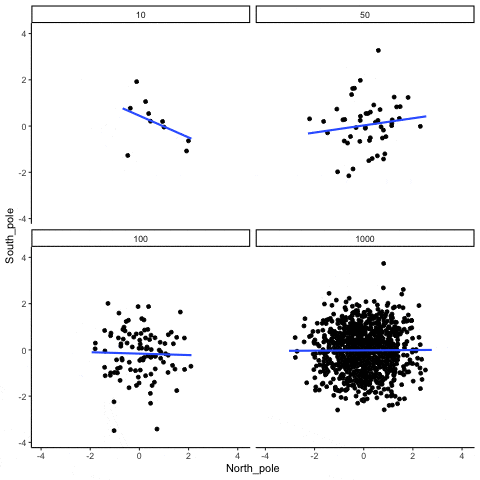
Of course, we don’t put much stock into a barely significant correlation from a single study with a small sample, because we understand the volatility of correlations in small samples. To reinforce this point, check out the following GIF made by Dr. Matt Crump. In all of the figures, the actual correlation within the population from which the samples are drawn is r = 0.
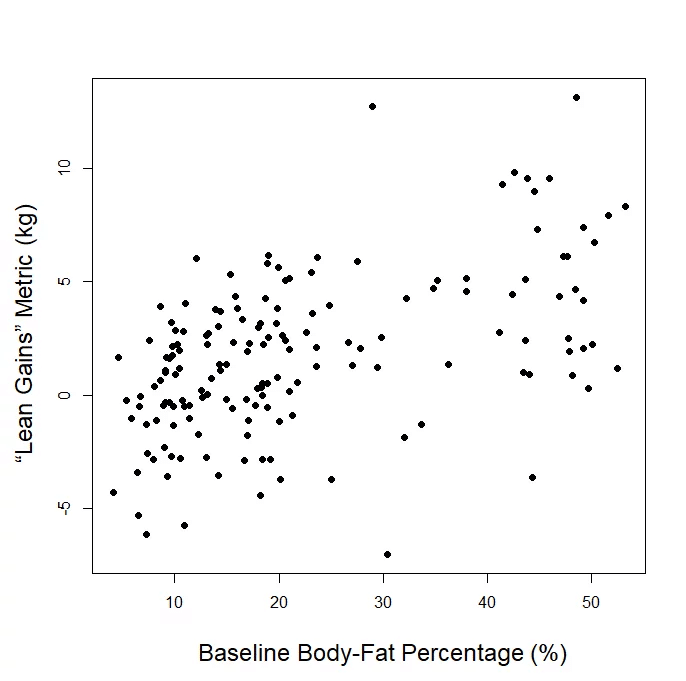
As we can see, correlations can be quite unstable when sample size is relatively small, but eventually stabilize as sample size increases. As such, it’s not particularly surprising that small studies assessing the correlation between baseline IL-6 and hypertrophy are a bit hit-or-miss. That doesn’t mean small-sample correlations are useless. It just means we’d like to see some pretty reliable replication from many small samples before making any large bets based on a correlation from a single study with less than 25 subjects. Menno’s argument fails to convincingly show that variations in IL-6 within the normal range are reliably predictive of hypertrophy potential, while also making no attempt whatsoever to indicate what body-fat level would be required to yield deleterious increases in IL-6, or whether or not such increases would be observed in young, physically active individuals who are training consistently. More importantly, why would a single significant negative correlation between baseline IL-6 and hypertrophy (Mitchell et al) tell us more about this topic than a single significant positive correlation between baseline body-fat and our body composition outcome of interest (Schoenfeld et al)? One study fails to provide the necessary data to make any connections between body-fat and body composition changes (Mitchell), but the other does (Schoenfeld), and the study that actually addresses the question shows that having higher body-fat at baseline led to better gains using our “lean gains” metric.
More importantly, our rebuttal essentially presented a challenge: be specific. Menno is alleging a relationship between variables that can all be quantified, but refuses to quantify them into an argument that actually holds meaning. Pick a biomarker, tell us what level of that biomarker is expected to blunt hypertrophy (or how much a given change in it would be expected to blunt hypertrophy), assign a magnitude to the degree of hypertrophy attenuation, and tell us what range of body-fat percentages would circumvent the issue. I’ll fall back on the almond metaphor: almonds have cyanide, cyanide kills people, so almonds kill people. High body-fat is linked to inflammation, inflammation is linked to blunted hypertrophy, so high body-fat blunts hypertrophy. Those two arguments contain the same amount of information, and at least one of them is laughably incorrect.
Furthermore, it’s somewhat disingenuous to state that we’re skeptical of the generalizability of the studies Menno cited simply because they were in older adults. For starters, it’s worth reiterating specifically why that’s a limitation. In younger, healthy individuals, inflammation is more-or-less reflective of the state of an individual at a certain point in time. Inflammation can go way up if someone’s engaging in a lot of pro-inflammatory behaviors, or go way down if they’re engaging in a lot of anti-inflammatory behaviors. In older adults, on the other hand, pro-inflammatory signaling is part of the positive feedback loop of cellular senescence. In other words, pro- and anti-inflammatory behaviors can still affect inflammation status at a given point in time in older adults, but there’s an essentially irreversible pattern of inflammation and pro-inflammatory signaling trending up over time, reflecting (and accelerating) cellular senescence (this is a bit of a simplification, but this also isn’t the article for getting into the nuances of the physiology of aging; the intro to this article explains this phenomenon, though, and if you want to read more about it, this pubmed query should point you in the right direction). In other words, studies in older adults may be suggesting that higher levels of inflammation have an independent, negative effect on hypertrophy, or they may merely be suggesting that older adults who are further down the pathway of senescence (people who are “older” on a cellular level, independent of chronological age) experience less hypertrophy than less senescent older adults, with inflammation primarily reflecting differences in senescence. Given these differences, it may not be safe to assume that inflammation-linked outcomes in older adults will generalize to younger adults.
Second, it’s worth reiterating that our critique went beyond differences in populations, to address what was measured, and how it was measured. A couple of the studies that Menno cited found that increases in pro-inflammatory markers (not baseline levels of pro-inflammatory markers) were negatively associated with hypertrophy (Orsatti et al. and Stec et al.); those studies aren’t informative here, because we know that resistance training generally decreases obesity-induced inflammation. One of the studies also assessed local changes (in the quads) of pro-inflammatory gene expression; that’s not the same thing as assessing systemic inflammation (the presumed obesity-induced state that is proposed to attenuate hypertrophy). Thus, compounding the fact that most of the studies Menno cited were conducted in populations where inflammatory markers need to be interpreted more cautiously, the inflammatory markers reported by some of the studies aren’t even relevant to the argument Menno is trying to make (that obesity-induced systemic inflammation will negatively affect hypertrophy).
Menno states: “Regarding protein balance, here too we differ in interpretation. There’s substantial support for anabolic resistance to food in overweight individuals, but in strength trainees, we can summarize the results as 1 study showing anabolic resistance and 1 study not showing it. Trexler & Nuckols are not concerned about this. I’m comfortable saying this is a negative trend in favor or not getting overweight. Significantly reduced muscle protein synthesis can be a big deal.”
Again, this fully ignores the substance of our rebuttal, but we do get closer to something resembling a magnitude. Apparently these data have a big, big impact, and impact things bigly, by a magnitude of 1 Big Unit (BU). Of course, the data that Menno is citing indicate that people with higher body-fat should be in a perpetual state of less-positive protein balance, and if we assume that is predictive of body composition over time, they should eventually end up having less body protein (lean mass) than leaner people, and that’s almost never the case in the countless studies that compare lean mass in people with lower versus higher body-fat. Plus, if we consider the single study with a resistance training component that Menno is leaning on and take it at face value, we have to assume that obese people who lift will gain slightly less muscle over time than obese people who do not lift, which seems hard to get on board with. In addition, it failed to find differences in systemic inflammation between the groups, which takes Menno’s most plausible mechanism off the table within that study. It’s also curious that the obese group had 32% more total lean mass and 32% greater leg lean mass than the leaner group, despite findings indicating that they must chronically be in more negative protein balance. All in all, it makes you start to wonder if these very acute indices of muscle protein synthesis can be used to make air-tight claims (or even somewhat confident conclusions) about longitudinal hypertrophy, and of course, they can’t.
Menno states: “Regarding recovery capacity, we seem to agree there’s actually considerable evidence overweight individuals have impaired recovery capacity. Trexler & Nuckols argue this is probably not a big deal, as the research is all in untrained individuals and animals. Again I’m not comfortable dismissing all this evidence. Trained individuals have better recovery capacity than untrained individuals, definitely, but would it completely wash out the detrimental effects of being overweight?”
Menno originally cited two human studies on this topic (one, two), both of which completely contradicted his claims about them. So, we built Menno’s case for him with more relevant data, then explained why it was a very weak case due to the major confounding effect of training status. Menno isn’t willing to dismiss all of the research we found for him; he seems to believe we should consider this to be important evidence linking body-fat levels to recovery capacity, then use it to make inferences about the relationship between body-fat and hypertrophy. But why is he so eager to dismiss the data in our subject-level meta-analysis, which actually looks directly at the relationship between body-fat percentage and hypertrophy?
Menno states: “On a side note, Trexler & Nuckols found 2 very cool studies that I was unaware of. These studies show that being underweight is bad for neuromuscular recovery as well. That’s exactly in line with my proposed optimum (inverted U) curve of body-fat percentage: it’s best to stay in the healthy range. This is yet another big limitation of Trexler & Nuckols’s unofficial meta-analysis: they try to find a linear effect of body-fat percentage, whereas they should be looking for a U-curve: there’s probably a positive effect at very low body-fat levels (they’re too lean) and a negative effect at high body-fat levels (they’re overweight). In the healthy and quite common range of body-fat, there will probably be no effect at all, e.g. at 10-15% for men.”
This is truly shocking, for two reasons. First, it seems to imply that we never paused to consider that excessively low body-fat would be unfavorable for hypertrophy. These are literal quotes from our original article:
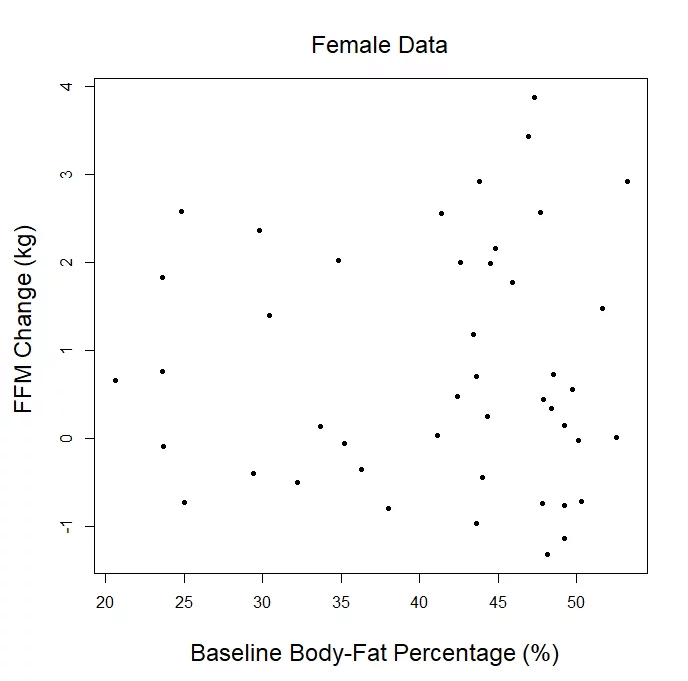
“These results indicate that people with relatively low and relatively high body-fat can gain fat-free mass to a pretty similar degree in response to resistance training. However, very lean people should accept that fat mass will probably need to increase a little bit to facilitate muscle gain, and they may need to get up to a slightly more comfortable body-fat level before lean mass gains really start to accumulate (for example, every single person under 8% body-fat at baseline had some degree of fat gain, and only one of them gained more than 1kg of fat-free mass).”
“As you progress from being super-shredded to extra-super-shredded, your likelihood of losing lean mass increases, and gains in fat-free mass are harder to come by (likely due to changes in hormone concentrations and physiological indicators of chronic energy status).”
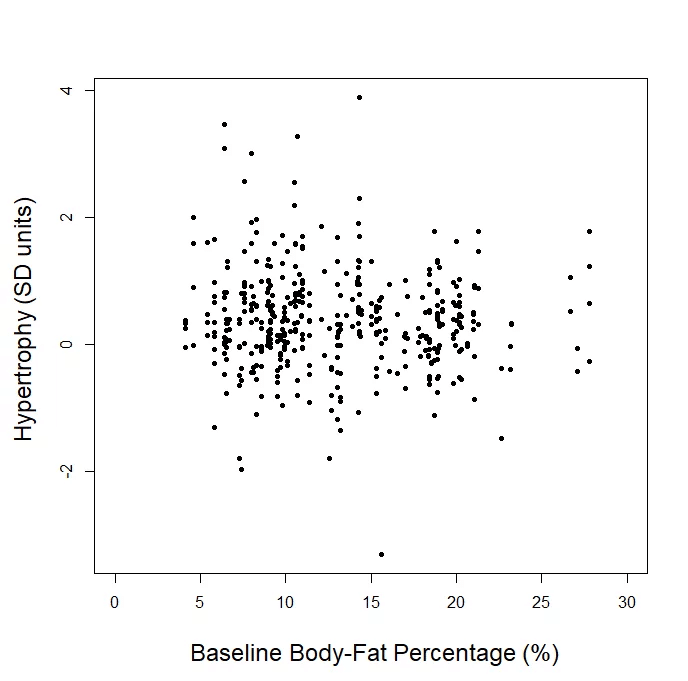
The second shocking part is that it implies an “inverted-U” shaped relationship, but we literally plotted the data for you. All of it. Totally raw, unadjusted, every single data point. We already provided the plots for our “lean gains” metric, direct measures of hypertrophy, and sex-specific changes in fat-free mass. If you can find an inverted U, by all means, draw it in. But bear in mind, in order for this to translate from an abstract art exercise to a meaningful argument, the data have to fit this U-shape tightly enough to argue that the trendline has useful predictive ability, and the downward slopes of the trendline at low and high body-fat levels have to be steep enough to argue that a small or moderate change in body-fat percentage translates to a meaningful change in hypertrophy potential. In reality, Menno’s argument is totally predicated on the assumptions that you either (A) did not read the article, or (B) don’t know what a U is shaped like.
Menno states: “Regarding hormonal health, again we differ in the degree of concern. Trexler & Nuckols argue it’s not a big deal for your hormonal health to be overweight. They rightfully show that on average, there’s indeed not a huge negative effect. However, as I showed, and as any fertility doctor should be able to attest to, for some people it is very much a big deal: they can develop clinical hypogonadism by becoming overweight and getting lean can solve the problem.
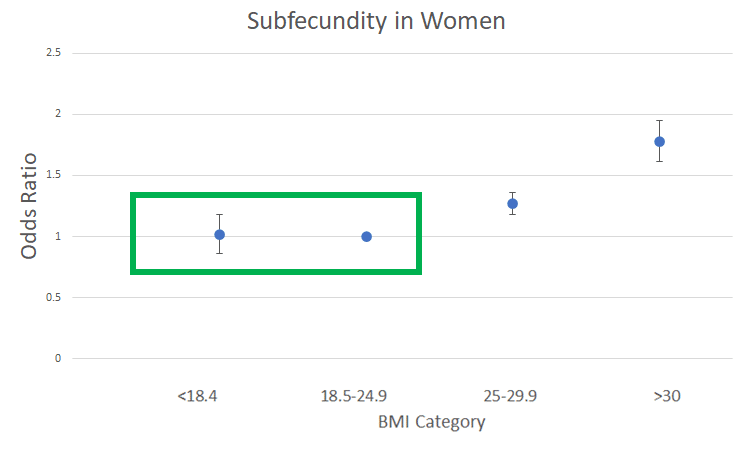
“On a technical note, their analysis of my citation of the subfecundity study is in my view again missing the larger point. To quote the researchers: “Among men and women with a BMI of 18.5 kg/m(2) or more, we found a [negative] dose-response relationship between increasing BMI group and subfecundity”. This relation disappeared at lower body-fat levels, so while we can debate how they classified the BMI categories and their conversion to BF%, the data clearly support that being overweight is bad for fertility and the trend is that the more overweight you are, the worse it is. I wouldn’t put too much stock into the exact numbers of 1 study.”
Menno claims to have “showed” that, “for some people,” the relationship between body-fat and sex hormones is a big deal for fertility. He first lays the foundation for this claim by vaguely appealing to authority (just ask any fertility doctor), and also by badly misinterpreting the subfecundity study he references. As we clearly laid out in our rebuttal, with actual numbers, the study showed that a woman going from a BMI of <18.4 to the 18.5-24.9 range actually has a decrease in subfecundity (good for fertility). Of course, this is a meaningless difference, as the odds ratio only decreased by 0.02, with a p-value of 0.80. We could not in good faith argue that a p-value of this magnitude means anything at all, and no one possibly could. In the study Menno cited, a man going from a BMI of <18.4 to the 18.5-24.9 range has a small increase in subfecundity (bad for fertility), but with an even higher p-value of 0.91. If you say these data indicate that the increase is meaningfully bad for men, then you literally must acknowledge the even stronger statistical argument that the increase is meaningfully good for women, but eventually you’d have to confront the fact that you’re making a terrible argument.
The quote from the abstract that he’s basing his interpretation on is actually pretty awesome. He previously accused us of foolishly missing the very important non-linear trend in our data (the “inverted U”), which is obviously not true. But here’s the kicker: Menno is ironically assuming linearity for this clearly non-linear relationship between BMI category and subfecundity. Here is the plotted trend for women, and the region in the green rectangle is being interpreted by Menno as a clinically meaningful increase:
Menno’s calculation implies that a BMI of 18.4 is the perfect BMI for fertility, while the trends in this data set couldn’t possibly be used to indicate any deleterious effect at a BMI below 25. Moral of the story: read more than the best quote from the abstract, and look at actual data sometimes. You could theoretically try to convert these BMIs to indicate that it would be a good idea, for fertility’s sake, based on this singular study, to keep body-fat below 18.5% for men and 29.3% for women. But that only relates to our p-ratio discussion if you assume that subfecundity is a decent proxy for hypertrophy, and I can’t imagine ever making such a bold leap.
For added humor, recall that Menno states: “…the data clearly support that being overweight is bad for fertility and the trend is that the more overweight you are, the worse it is. I wouldn’t put too much stock into the exact numbers of 1 study.”
This whole line of argument about fertility is fully uninformative for the actual topic at hand (body-fat and hypertrophy). The only reason we addressed it is because it inexplicably became part of his rebuttal, and he quoted an exact number that was “optimal” for fertility. Not a range, not a ballpark estimate, but an exact number down to the tenths digit, derived from a single (misinterpreted) study. He stated: “For a 21-year-old, the optimal fat level for fertility corresponds to a body-fat percentage of just 10.7% for untrained men and 21.5% for women based on this.” The only reason we corrected his pretty flagrant mistake was because he so boldly and confidently stated these specific numbers based on a single study, and we were concerned that (A) that degree of confidence was unwarranted, (B) the idea of translating an entire BMI category to a single body-fat percentage, down to the tenths digit, seemed unjustifiable, and (C) even if you felt good about doing that, you shouldn’t list a number that is incorrect. In essence, Menno listed a very specific number, we implored readers not to take it seriously, and his most recent response seems to reprimand us for even considering the possibility of taking his claim seriously.
In Menno’s conclusion, he states: “I think Trexler & Nuckols are falling a bit into the trap of mistaking absence of evidence for evidence of absence.”
This is a complete misrepresentation without a granule of truth to it. Our rebuttal directly states:
“I take no issue with people turning hunches and anecdotes into practical recommendations, as long as they don’t claim that the recommendation is based on robust scientific evidence. I don’t have enough evidence to assert that this whole p-ratio hypothesis cannot exist, my article simply indicates that I can’t find any convincing evidence to support it, and I can find some indirect evidence that seems to contradict it. My article can’t be used to permanently and conclusively “debunk” the concept, but at the very least, it should encourage people to discuss the concept in a way that effectively conveys the tremendous amount of speculation and surprising lack of support that the concept rests upon.”
We very explicitly stated that we are not conflating absence of evidence with evidence of absence. We simply gathered all of the most applicable and generalizable evidence on the topic, carefully analyzed it, and we were able to find plenty of evidence contradicting the old p-ratio hypothesis and very little evidence supporting it. Upon weighing the strength and generalizability of the scientific evidence for both sides of the argument, we tentatively conclude that the old p-ratio hypothesis, which represents a rejection of the null hypothesis, is very difficult to justify based on the evidence available. If we fell into a trap, the trap is called “evidence-based practice,” which is a much more hospitable trap than the confirmation bias that seems to have ensnared Menno.
Unanswered Questions
Menno’s initial rebuttal questioned our meta-analysis for failing to control for a variety of variables. We provided updated analyses to directly alleviate every single one of the concerns; this was fully ignored in his most recent response. Further, our rebuttal posed a few very specific questions:
How can we assert that high body-fat impairs protein balance, but is required for reaching your maximal muscular potential?
What is the actual, quantified relationship between body-fat, inflammation, and hypertrophy impairment, using any numbers whatsoever?
What is the actual, quantified relationship between body-fat, hormones influencing muscle mass accretion, and hypertrophy impairment, using any numbers whatsoever?
If the claim is that high body-fat impairs hypertrophy, why should we discard the relationship between body-fat and hypertrophy in longitudinal hypertrophy studies covering a huge range of body-fat levels?
How are studies about postmenopausal inflammation or rodents with gene mutations supposed to provide more direct answers to this question when compared to longitudinal studies that measured the predictor variable, measured the outcome variable, and applied a robust resistance training stimulus?
Unfortunately, the full depth of Menno’s recent response essentially boils down to asserting that inflammation seems bad, the rodent study provides proof of concept if you ignore one of the groups or shift the blame back to insulin resistance instead of body-fat percentage, and that his claim about subfecundity shouldn’t be taken seriously, but feels good in spirit. After unjustifiably disregarding the most direct evidence, thereby asserting that there is no evidence, it closes by erroneously suggesting that we’re conflating the imaginary absence of evidence as evidence of absence. Menno has done plenty of theorizing and handwaving, but our substantive questions remain fully unanswered.
A Tale of Two Approaches
When Menno posted his rebuttal to our initial article, he mostly used recycled links from his old articles, and failed to actually quantify any of the easily quantifiable claims he was making. In turn, we responded with a full and thorough response. For every “limitation” of our subject-level meta-analysis, we responded with an adjusted analysis to alleviate the concern. For every vague, unquantified argument about how inflammation should do this or testosterone should do that, we decisively rejected the arguments based on fully quantified, and heavily referenced arguments. Our rebuttal was over 16,000 words, with countless citations and nuanced data analysis with new subsets, new covariates, new hierarchical nesting of random effects, new predictor variables, and new outcome variables. It wasn’t a fishing expedition to prove our point; we chased down every wild goose that Menno encouraged us to catch, and none of them brought us any closer to support for his claims. In contrast, look at Menno’s response to our rebuttal. There are no references. There are no calculations. The only response to our thorough re-analysis of our meta-analytic data involves an extremely creative interpretation of what the letter U is shaped like. The only numbers, aside from quantifying the number of studies that say this or the number of studies that found that, include a badly misinterpreted BMI value, and a completely arbitrary “ideal body-fat range” of 10-15% that is based on no evidence whatsoever. Unless you count “big” as a numerical expression.
We recognize that this response is fairly snarky, but we think some degree of snark is justified. We know Menno is capable of high-quality work and nuanced thought, and as such, we found both his initial rebuttal and his response – which misrepresented our points and analyses, misrepresented his own citations, and leaned on a lot of hand-waving in place of quantifiable claims – to be quite intellectually insulting.
At this point, we feel like we’ve pretty thoroughly dissected the research relevant to this topic and addressed all counterpoints presented. So, we would encourage readers to evaluate the topic with an open mind, and let the evidence guide you to your own conclusions until more data become available.